Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin
- PMID: 23082970
- PMCID: PMC3579771
- DOI: 10.1021/ja307974e
Calcium ion gradients modulate the zinc affinity and antibacterial activity of human calprotectin
Abstract
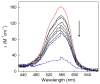
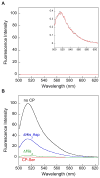
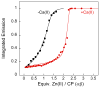
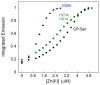
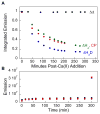
Calprotectin (CP) is an antimicrobial protein produced and released by neutrophils that inhibits the growth of pathogenic microorganisms by sequestering essential metal nutrients in the extracellular space. In this work, spectroscopic and thermodynamic metal-binding studies are presented to delineate the zinc-binding properties of CP. Unique optical absorption and EPR spectroscopic signatures for the interfacial His(3)Asp and His(4) sites of human calprotectin are identified by using Co(II) as a spectroscopic probe. Zinc competition titrations employing chromophoric Zn(II) indicators provide a 2:1 Zn(II):CP stoichiometry, confirm that the His(3)Asp and His(4) sites of CP coordinate Zn(II), and reveal that the Zn(II) affinity of both sites is calcium-dependent. The calcium-insensitive Zn(II) competitor ZP4 affords dissociation constants of K(d1) = 133 ± 58 pM and K(d2) = 185 ± 219 nM for CP in the absence of Ca(II). These values decrease to K(d1) ≤ 10 pM and K(d2) ≤ 240 pM in the presence of excess Ca(II). The K(d1) and K(d2) values are assigned to the His(3)Asp and His(4) sites, respectively. In vitro antibacterial activity assays indicate that the metal-binding sites and Ca(II)-replete conditions are required for CP to inhibit the growth of both Gram-negative and -positive bacteria. Taken together, these data provide a working model whereby calprotectin responds to physiological Ca(II) gradients to become a potent Zn(II) chelator in the extracellular space.
Figures





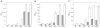
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

