Oocyte-somatic cells interactions, lessons from evolution
- PMID: 23083410
- PMCID: PMC3532176
- DOI: 10.1186/1471-2164-13-560
Oocyte-somatic cells interactions, lessons from evolution
Abstract
Background: Despite the known importance of somatic cells for oocyte developmental competence acquisition, the overall mechanisms underlying the acquisition of full developmental competence are far from being understood, especially in non-mammalian species. The present work aimed at identifying key molecular signals from somatic origin that would be shared by vertebrates.
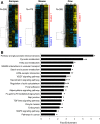
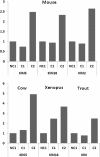
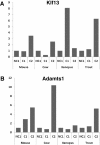
Results: Using a parallel transcriptomic analysis in 4 vertebrate species - a teleost fish, an amphibian, and two mammals - at similar key steps of developmental competence acquisition, we identified a large number of species-specific differentially expressed genes and a surprisingly high number of orthologous genes exhibiting similar expression profiles in the 3 tetrapods and in the 4 vertebrates. Among the evolutionary conserved players participating in developmental competence acquisition are genes involved in key processes such as cellular energy metabolism, cell-to-cell communications, and meiosis control. In addition, we report many novel molecular actors from somatic origin that have never been studied in the vertebrate ovary. Interestingly, a significant number of these new players actively participate in Drosophila oogenesis.
Conclusions: Our study provides a comprehensive overview of evolutionary-conserved mechanisms from somatic origin participating in oocyte developmental competence acquisition in 4 vertebrates. Together our results indicate that despite major differences in ovarian follicular structure, some of the key players from somatic origin involved in oocyte developmental competence acquisition would be shared, not only by vertebrates, but also by metazoans. The conservation of these mechanisms during vertebrate evolution further emphasizes the important contribution of the somatic compartment to oocyte quality and paves the way for future investigations aiming at better understanding what makes a good egg.
Figures




Similar articles
-
Comparative transcriptomic analysis of follicle-enclosed oocyte maturational and developmental competence acquisition in two non-mammalian vertebrates.BMC Genomics. 2010 Jan 8;11:18. doi: 10.1186/1471-2164-11-18. BMC Genomics. 2010. PMID: 20059772 Free PMC article.
-
Identification of differentially expressed miRNAs and their potential targets during fish ovarian development.Biol Reprod. 2013 May 23;88(5):128. doi: 10.1095/biolreprod.112.105361. Print 2013 May. Biol Reprod. 2013. PMID: 23595902
-
In silico identification and molecular characterization of genes predominantly expressed in the fish oocyte.BMC Genomics. 2008 Oct 23;9:499. doi: 10.1186/1471-2164-9-499. BMC Genomics. 2008. PMID: 18947432 Free PMC article.
-
All in one - integrating cell polarity, meiosis, mitosis and mechanical forces in early oocyte differentiation in vertebrates.Int J Dev Biol. 2017;61(3-4-5):179-193. doi: 10.1387/ijdb.170030ye. Int J Dev Biol. 2017. PMID: 28621416 Review.
-
Oocyte maturation: gamete-somatic cells interactions, meiotic resumption, cytoskeletal dynamics and cytoplasmic reorganization.Hum Reprod Update. 2015 Jul-Aug;21(4):427-54. doi: 10.1093/humupd/dmv011. Epub 2015 Mar 4. Hum Reprod Update. 2015. PMID: 25744083 Review.
Cited by
-
Ligands, Receptors, and Transcription Factors that Mediate Inter-Cellular and Intra-Cellular Communication during Ovarian Follicle Development.Reprod Sci. 2020 Feb;27(2):690-703. doi: 10.1007/s43032-019-00075-8. Epub 2020 Jan 14. Reprod Sci. 2020. PMID: 31939199 Free PMC article.
-
LncGSAR Controls Ovarian Granulosa Cell Steroidogenesis via Sponging MiR-125b to Activate SCAP/SREBP Pathway.Int J Mol Sci. 2022 Oct 12;23(20):12132. doi: 10.3390/ijms232012132. Int J Mol Sci. 2022. PMID: 36293007 Free PMC article.
-
Long Non-Coding RNA GDAR Regulates Ovine Granulosa Cells Apoptosis by Affecting the Expression of Apoptosis-Related Genes.Int J Mol Sci. 2022 May 6;23(9):5183. doi: 10.3390/ijms23095183. Int J Mol Sci. 2022. PMID: 35563579 Free PMC article.
-
Dynamic genome-scale cell-specific metabolic models reveal novel inter-cellular and intra-cellular metabolic communications during ovarian follicle development.BMC Bioinformatics. 2019 Jun 10;20(1):307. doi: 10.1186/s12859-019-2825-2. BMC Bioinformatics. 2019. PMID: 31182013 Free PMC article.
-
Protein Palmitoylation in Bovine Ovarian Follicle.Int J Mol Sci. 2021 Oct 29;22(21):11757. doi: 10.3390/ijms222111757. Int J Mol Sci. 2021. PMID: 34769186 Free PMC article.
References
-
- Mermillod P, Dalbies-Tran R, Uzbekova S, Thelie A, Traverso JM, Perreau C, Papillier P, Monget P. Factors affecting oocyte quality: who is driving the follicle? Reproduction in domestic animals = Zuchthygiene. 2008;43(Suppl 2):393–400. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

