Bacterial thermotaxis by speed modulation
- PMID: 23083711
- PMCID: PMC3475339
- DOI: 10.1016/j.bpj.2012.09.005
Bacterial thermotaxis by speed modulation
Abstract
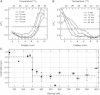
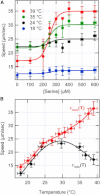
Naturally occurring gradients often extend over relatively long distances such that their steepness is too small for bacteria to detect. We studied the bacterial behavior in such thermal gradients. We find that bacteria migrate along shallow thermal gradients due to a change in their swimming speed resulting from the effect of temperature on the intracellular pH, which also depends on the chemical environment. When nutrients are scarce in the environment the bacteria's intracellular pH decreases with temperature. As a result, the swimming speed of the bacteria decreases with temperature, which causes them to slowly drift toward the warm end of the thermal gradient. However, when serine is added to the medium at concentrations >300 μM, the intracellular pH increases causing the swimming speed to increase continuously with temperature, and the bacteria to drift toward the cold end of the temperature gradient. This directional migration is not a result of bacterial thermotaxis in the classical sense, because the steepness of the gradients applied is below the sensing threshold of bacteria. Nevertheless, our results show that the directional switch requires the presence of the bacterial sensing receptors. This seems to be due to the involvement of the receptors in regulating the intracellular pH.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
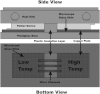



References
-
- Berg H.C. Springer; New York, NY: 2003. E. coli in Motion.
-
- Baker M.D., Wolanin P.M., Stock J.B. Signal transduction in bacterial chemotaxis. Bioessays. 2006;28:9–22. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

