Design of peptide-membrane interactions to modulate single-file water transport through modified gramicidin channels
- PMID: 23083713
- PMCID: PMC3475385
- DOI: 10.1016/j.bpj.2012.08.059
Design of peptide-membrane interactions to modulate single-file water transport through modified gramicidin channels
Abstract
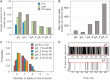
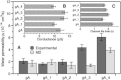
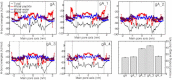
Water permeability through single-file channels is affected by intrinsic factors such as their size and polarity and by external determinants like their lipid environment in the membrane. Previous computational studies revealed that the obstruction of the channel by lipid headgroups can be long-lived, in the range of nanoseconds, and that pore-length-matching membrane mimetics could speed up water permeability. To test the hypothesis of lipid-channel interactions modulating channel permeability, we designed different gramicidin A derivatives with attached acyl chains. By combining extensive molecular-dynamics simulations and single-channel water permeation measurements, we show that by tuning lipid-channel interactions, these modifications reduce the presence of lipid headgroups in the pore, which leads to a clear and selective increase in their water permeability.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Lee A.G. How lipids and proteins interact in a membrane: a molecular approach. Mol. Biosyst. 2005;1:203–212. - PubMed
-
- Nyholm T.K.M., Ozdirekcan S., Killian J.A. How protein transmembrane segments sense the lipid environment. Biochemistry. 2007;46:1457–1465. - PubMed
-
- Jensen M.O., Mouritsen O.G. Lipids do influence protein function-the hydrophobic matching hypothesis revisited. Biochim. Biophys. Acta. 2004;1666:205–226. - PubMed
-
- Valiyaveetil F.I., Zhou Y., MacKinnon R. Lipids in the structure, folding, and function of the KcsA K+ channel. Biochemistry. 2002;41:10771–10777. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

