Parametric functional maps of visual inputs to the tectum
- PMID: 23083735
- PMCID: PMC4516722
- DOI: 10.1016/j.neuron.2012.08.040
Parametric functional maps of visual inputs to the tectum
Erratum in
- Neuron. 2013 Mar 6;77(5):992
Abstract
How features of the visual scene are encoded in the population activity of retinal ganglion cells (RGCs) targeting specific regions of the brain is not well understood. To address this, we have used a genetically encoded reporter of presynaptic function (SyGCaMP3) to record visually evoked activity in the population of RGC axons innervating the zebrafish tectum. Using unbiased voxel-wise analysis of SyGCaMP3 signals, we identify three subtypes of direction-selective and two subtypes of orientation-selective retinal input. Composite parametric functional maps generated across many larvae show laminar segregation of direction- and orientation-selective responses and unexpected retinotopic biases in the distribution of functional subtypes. These findings provide a systematic description of the form, organization, and dimensionality of visual inputs to the brain and will serve as a platform for understanding emergent properties in tectal circuits associated with visually driven behavior.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
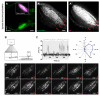


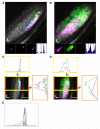
Comment in
-
What the fish's eye tells the fish's brain.Neuron. 2012 Oct 18;76(2):257-9. doi: 10.1016/j.neuron.2012.10.006. Epub 2012 Oct 17. Neuron. 2012. PMID: 23083728
Similar articles
-
Precise lamination of retinal axons generates multiple parallel input pathways in the tectum.J Neurosci. 2013 Mar 13;33(11):5027-39. doi: 10.1523/JNEUROSCI.4990-12.2013. J Neurosci. 2013. PMID: 23486973 Free PMC article.
-
Lamination Speeds the Functional Development of Visual Circuits.Neuron. 2015 Dec 2;88(5):999-1013. doi: 10.1016/j.neuron.2015.10.020. Epub 2015 Nov 19. Neuron. 2015. PMID: 26607001 Free PMC article.
-
A Three-Layer Network Model of Direction Selective Circuits in the Optic Tectum.Front Neural Circuits. 2017 Nov 21;11:88. doi: 10.3389/fncir.2017.00088. eCollection 2017. Front Neural Circuits. 2017. PMID: 29209178 Free PMC article.
-
Direction selectivity in the visual system of the zebrafish larva.Front Neural Circuits. 2013 Jun 18;7:111. doi: 10.3389/fncir.2013.00111. eCollection 2013. Front Neural Circuits. 2013. PMID: 23785314 Free PMC article. Review.
-
The Visual Systems of Zebrafish.Annu Rev Neurosci. 2024 Aug;47(1):255-276. doi: 10.1146/annurev-neuro-111020-104854. Epub 2024 Jul 1. Annu Rev Neurosci. 2024. PMID: 38663429 Review.
Cited by
-
Whole-brain functional imaging at cellular resolution using light-sheet microscopy.Nat Methods. 2013 May;10(5):413-20. doi: 10.1038/nmeth.2434. Epub 2013 Mar 18. Nat Methods. 2013. PMID: 23524393
-
Optic tectal superficial interneurons detect motion in larval zebrafish.Protein Cell. 2019 Apr;10(4):238-248. doi: 10.1007/s13238-018-0587-7. Epub 2018 Nov 12. Protein Cell. 2019. PMID: 30421356 Free PMC article.
-
Functional segregation of retinal ganglion cell projections to the optic tectum of rainbow trout.J Neurophysiol. 2015 Nov;114(5):2703-17. doi: 10.1152/jn.00440.2015. Epub 2015 Sep 2. J Neurophysiol. 2015. PMID: 26334009 Free PMC article.
-
Molecular features distinguish ten neuronal types in the mouse superficial superior colliculus.J Comp Neurol. 2016 Aug 1;524(11):2300-21. doi: 10.1002/cne.23952. Epub 2016 Jan 26. J Comp Neurol. 2016. PMID: 26713509 Free PMC article.
-
Orientation-Selective Retinal Circuits in Vertebrates.Front Neural Circuits. 2018 Feb 7;12:11. doi: 10.3389/fncir.2018.00011. eCollection 2018. Front Neural Circuits. 2018. PMID: 29467629 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
