Slow cortical dynamics and the accumulation of information over long timescales
- PMID: 23083743
- PMCID: PMC3517908
- DOI: 10.1016/j.neuron.2012.08.011
Slow cortical dynamics and the accumulation of information over long timescales
Erratum in
- Neuron. 2012 Nov 8;76(3):668
Abstract
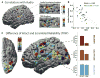
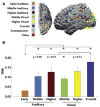
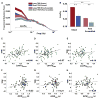
Making sense of the world requires us to process information over multiple timescales. We sought to identify brain regions that accumulate information over short and long timescales and to characterize the distinguishing features of their dynamics. We recorded electrocorticographic (ECoG) signals from individuals watching intact and scrambled movies. Within sensory regions, fluctuations of high-frequency (64-200 Hz) power reliably tracked instantaneous low-level properties of the intact and scrambled movies. Within higher order regions, the power fluctuations were more reliable for the intact movie than the scrambled movie, indicating that these regions accumulate information over relatively long time periods (several seconds or longer). Slow (<0.1 Hz) fluctuations of high-frequency power with time courses locked to the movies were observed throughout the cortex. Slow fluctuations were relatively larger in regions that accumulated information over longer time periods, suggesting a connection between slow neuronal population dynamics and temporally extended information processing.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



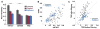
References
-
- Ashburner J. A fast diffeomorphic image registration algorithm. NeuroImage. 2007;38:95–113. - PubMed
-
- Benjamini Y, Hochberg Y. Controlling the false discovery rate—a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57:289–300.
-
- Brody CD, Romo R, Kepecs A. Basic mechanisms for graded persistent activity: discrete attractors, continuous attractors, and dynamic representations. Curr Opin Neurobiol. 2003;13:204–211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

