Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer
- PMID: 23083876
- PMCID: PMC3856891
- DOI: 10.1016/j.juro.2012.08.030
Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer
Abstract
Purpose: Recently, a new renal cell cancer syndrome has been linked to germline mutation of multiple subunits (SDHB/C/D) of the Krebs cycle enzyme, succinate dehydrogenase. We report our experience with the diagnosis, evaluation and treatment of this novel form of hereditary kidney cancer.
Materials and methods: Patients with suspected hereditary kidney cancer were enrolled on a National Cancer Institute institutional review board approved protocol to study inherited forms of kidney cancer. Individuals from families with germline SDHB, SDHC and SDHD mutations, and kidney cancer underwent comprehensive clinical and genetic evaluation.
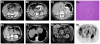
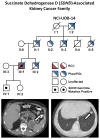
Results: A total of 14 patients from 12 SDHB mutation families were evaluated. Patients presented with renal cell cancer at an early age (33 years, range 15 to 62), metastatic kidney cancer developed in 4 and some families had no manifestation other than kidney tumors. An additional family with 6 individuals found to have clear cell renal cell cancer that presented at a young average age (47 years, range 40 to 53) was identified with a germline SDHC mutation (R133X) Metastatic disease developed in 2 of these family members. A patient with a history of carotid body paragangliomas and an aggressive form of kidney cancer was evaluated from a family with a germline SDHD mutation.
Conclusions: SDH mutation associated renal cell carcinoma can be an aggressive type of kidney cancer, especially in younger individuals. Although detection and management of early tumors is most often associated with a good outcome, based on our initial experience with these patients and our long-term experience with hereditary leiomyomatosis and renal cell carcinoma, we recommend careful surveillance of patients at risk for SDH mutation associated renal cell carcinoma and wide surgical excision of renal tumors.
Copyright © 2012 American Urological Association Education and Research, Inc. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406. - PubMed
-
- Grubb RL, III, Franks ME, Toro J, et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177:2074. - PubMed
-
- Warburg O, Posener K, Negelein E. Metabolism of the Carcinoma Cell. Biochem Z. 1924;152:309.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

