Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease
- PMID: 23084357
- PMCID: PMC3495160
- DOI: 10.1016/j.immuni.2012.10.003
Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease
Abstract
The mammalian intestine harbors trillions of beneficial commensal bacteria that are essential for the development of the immune system and for maintenance of physiologic processes in multiple organs. However, numerous chronic infectious, inflammatory, and metabolic diseases in humans have been associated with alterations in the composition or localization of commensal bacteria that result in dysregulated host-commensal bacteria relationships. The mammalian immune system plays an essential role in regulating the acquisition, composition, and localization of commensal bacteria in the intestine. Emerging research has implicated innate lymphoid cells (ILCs) as a critical immune cell population that orchestrates some of these host-commensal bacteria relationships that can impact immunity, inflammation, and tissue homeostasis in the intestine. This review will discuss reciprocal interactions between intestinal commensal bacteria and ILCs in the context of health and disease.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

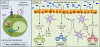

References
-
- Amar J, Serino M, Lange C, Chabo C, Iacovoni J, Mondot S, Lepage P, Klopp C, Mariette J, Bouchez O, et al. Involvement of tissue bacteria in the onset of diabetes in humans: evidence for a concept. Diabetologia. 2011 - PubMed
-
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. - PubMed
-
- Bouskra D, Brezillon C, Berard M, Werts C, Varona R, Boneca IG, Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI074878/AI/NIAID NIH HHS/United States
- AI061570/AI/NIAID NIH HHS/United States
- T32-AI055428/AI/NIAID NIH HHS/United States
- AI074878/AI/NIAID NIH HHS/United States
- AI087990/AI/NIAID NIH HHS/United States
- R21 AI087990/AI/NIAID NIH HHS/United States
- AI095608/AI/NIAID NIH HHS/United States
- R21 AI083480/AI/NIAID NIH HHS/United States
- U01 AI095608/AI/NIAID NIH HHS/United States
- R01 AI061570/AI/NIAID NIH HHS/United States
- AI097333/AI/NIAID NIH HHS/United States
- T32 AI055428/AI/NIAID NIH HHS/United States
- R01 AI095466/AI/NIAID NIH HHS/United States
- DP5OD012116/OD/NIH HHS/United States
- DP5 OD012116/OD/NIH HHS/United States
- AI083480/AI/NIAID NIH HHS/United States
- R01 AI102942/AI/NIAID NIH HHS/United States
- R01 AI097333/AI/NIAID NIH HHS/United States
- AI095466/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources

