Host targeting of virulence determinants and phosphoinositides in blood stage malaria parasites
- PMID: 23084821
- PMCID: PMC3501602
- DOI: 10.1016/j.pt.2012.09.004
Host targeting of virulence determinants and phosphoinositides in blood stage malaria parasites
Abstract
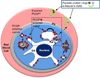
Blood stage malaria parasites target a 'secretome' of hundreds of proteins including virulence determinants containing a host (cell) targeting (HT) signal, to human erythrocytes. Recent studies reveal that the export mechanism is due to the HT signal binding to the lipid phosphatidylinositol-3-phosphate [PI(3)P] in the parasite endoplasmic reticulum (ER). An aspartic protease plasmepsin V which cleaves a specialized form of the HT signal was previously thought to be the export mechanism, but is now recognized as a dedicated peptidase that cleaves the signal anchor subsequent to PI(3)P binding. We discuss a model of PI(3)P-dependent targeting and PI(3)P biology of a major human pathogen.
Published by Elsevier Ltd.
Figures



Similar articles
-
Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell.Cell. 2012 Jan 20;148(1-2):201-12. doi: 10.1016/j.cell.2011.10.051. Cell. 2012. PMID: 22265412 Free PMC article.
-
PI(3)P-independent and -dependent pathways function together in a vacuolar translocation sequence to target malarial proteins to the host erythrocyte.Mol Biochem Parasitol. 2012 Oct;185(2):106-13. doi: 10.1016/j.molbiopara.2012.07.004. Epub 2012 Jul 22. Mol Biochem Parasitol. 2012. PMID: 22828070 Free PMC article.
-
Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte.Nature. 2010 Feb 4;463(7281):632-6. doi: 10.1038/nature08726. Nature. 2010. PMID: 20130644 Free PMC article.
-
Eukaryotic virulence determinants utilize phosphoinositides at the ER and host cell surface.Trends Microbiol. 2013 Mar;21(3):145-56. doi: 10.1016/j.tim.2012.12.004. Epub 2013 Jan 30. Trends Microbiol. 2013. PMID: 23375057 Free PMC article. Review.
-
Cerebral malaria pathogenesis: revisiting parasite and host contributions.Future Microbiol. 2012 Feb;7(2):291-302. doi: 10.2217/fmb.11.155. Future Microbiol. 2012. PMID: 22324996 Review.
Cited by
-
Genome analysis of Excretory/Secretory proteins in Taenia solium reveals their Abundance of Antigenic Regions (AAR).Sci Rep. 2015 May 19;5:9683. doi: 10.1038/srep09683. Sci Rep. 2015. PMID: 25989346 Free PMC article.
-
Why do malaria parasites increase host erythrocyte permeability?Trends Parasitol. 2014 Mar;30(3):151-9. doi: 10.1016/j.pt.2014.01.003. Epub 2014 Feb 5. Trends Parasitol. 2014. PMID: 24507014 Free PMC article. Review.
-
Whole genome sequencing identifies novel mutations in malaria parasites resistant to artesunate (ATN) and to ATN + mefloquine combination.Front Cell Infect Microbiol. 2024 Mar 1;14:1353057. doi: 10.3389/fcimb.2024.1353057. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38495651 Free PMC article.
-
The Plasmodium berghei translocon of exported proteins reveals spatiotemporal dynamics of tubular extensions.Sci Rep. 2015 Jul 29;5:12532. doi: 10.1038/srep12532. Sci Rep. 2015. PMID: 26219962 Free PMC article.
-
The systematic analysis of protein-lipid interactions comes of age.Nat Rev Mol Cell Biol. 2015 Dec;16(12):753-61. doi: 10.1038/nrm4080. Epub 2015 Oct 28. Nat Rev Mol Cell Biol. 2015. PMID: 26507169 Review.
References
-
- Collins WE. Plasmodium knowlesi: a malaria parasite of monkeys and humans. Annual review of entomology. 2012;57:107–121. - PubMed
-
- Miller LH, et al. The pathogenic basis of malaria. Nature. 2002;415:673–679. - PubMed
-
- Haldar K, Mohandas N. Erythrocyte remodeling by malaria parasites. Curr Opin Hematol. 2007;14:203–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

