Innate immune sensing of HIV-1 by dendritic cells
- PMID: 23084911
- PMCID: PMC3619430
- DOI: 10.1016/j.chom.2012.10.002
Innate immune sensing of HIV-1 by dendritic cells
Abstract
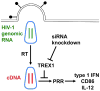
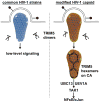
HIV-1-specific antibodies and CD8(+) cytotoxic T cells are detected in most HIV-1-infected people, yet HIV-1 infection is not eradicated. Contributing to the failure to mount a sterilizing immune response may be the inability of antigen-presenting dendritic cells (DCs) to sense HIV-1 during acute infection, and thus the inability to effectively prime naive, HIV-1-specific T cells. Recent findings related to DC-expressed innate immune factors including SAMHD1, TREX1, and TRIM5 provide a molecular basis for understanding why DCs fail to adequately sense invasion by this deadly pathogen and suggest experimental approaches to improve T cell priming to HIV-1 in prophylactic vaccination protocols.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Dendritic cell-lymphocyte cross talk downregulates host restriction factor SAMHD1 and stimulates HIV-1 replication in dendritic cells.J Virol. 2014 May;88(9):5109-21. doi: 10.1128/JVI.03057-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574390 Free PMC article.
-
Unmasking immune sensing of retroviruses: interplay between innate sensors and host effectors.Cytokine Growth Factor Rev. 2014 Dec;25(6):657-68. doi: 10.1016/j.cytogfr.2014.08.006. Epub 2014 Sep 3. Cytokine Growth Factor Rev. 2014. PMID: 25240798 Review.
-
HIV type 1 infection of plasmacytoid and myeloid dendritic cells is restricted by high levels of SAMHD1 and cannot be counteracted by Vpx.AIDS Res Hum Retroviruses. 2014 Feb;30(2):195-203. doi: 10.1089/AID.2013.0119. Epub 2013 Sep 4. AIDS Res Hum Retroviruses. 2014. PMID: 23924154 Free PMC article.
-
SAMHD1 restricts HIV-1 cell-to-cell transmission and limits immune detection in monocyte-derived dendritic cells.J Virol. 2013 Mar;87(5):2846-56. doi: 10.1128/JVI.02514-12. Epub 2012 Dec 26. J Virol. 2013. PMID: 23269793 Free PMC article.
-
SAMHD1 in Retroviral Restriction and Innate Immune Sensing--Should We Leash the Hound?Curr HIV Res. 2016;14(3):225-34. doi: 10.2174/1570162x14999160224102515. Curr HIV Res. 2016. PMID: 26957197 Review.
Cited by
-
Tetramerization of SAMHD1 is required for biological activity and inhibition of HIV infection.J Biol Chem. 2013 Apr 12;288(15):10406-17. doi: 10.1074/jbc.M112.443796. Epub 2013 Feb 20. J Biol Chem. 2013. PMID: 23426366 Free PMC article.
-
Dendritic cell dysregulation during HIV-1 infection.Immunol Rev. 2013 Jul;254(1):170-89. doi: 10.1111/imr.12082. Immunol Rev. 2013. PMID: 23772620 Free PMC article. Review.
-
Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses.Science. 2013 Aug 23;341(6148):903-6. doi: 10.1126/science.1240933. Epub 2013 Aug 8. Science. 2013. PMID: 23929945 Free PMC article.
-
Dendritic cell-lymphocyte cross talk downregulates host restriction factor SAMHD1 and stimulates HIV-1 replication in dendritic cells.J Virol. 2014 May;88(9):5109-21. doi: 10.1128/JVI.03057-13. Epub 2014 Feb 26. J Virol. 2014. PMID: 24574390 Free PMC article.
-
The TLR7/IRF-5 axis sensitizes memory CD4+ T cells to Fas-mediated apoptosis during HIV-1 infection.JCI Insight. 2023 Jul 10;8(13):e167329. doi: 10.1172/jci.insight.167329. JCI Insight. 2023. PMID: 37227774 Free PMC article.
References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, Montefiori DC, O’Connor DH, Davis BT, Lee PK, et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420:434–439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

