Gut-derived serotonin is a multifunctional determinant to fasting adaptation
- PMID: 23085101
- PMCID: PMC3696514
- DOI: 10.1016/j.cmet.2012.09.014
Gut-derived serotonin is a multifunctional determinant to fasting adaptation
Abstract
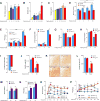
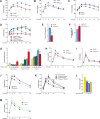
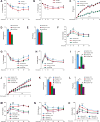
Energy release from cellular storage is mandatory for survival during fasting. This is achieved through lipolysis and liver gluconeogenesis. We show here that in the mouse, gut-derived serotonin (GDS) is upregulated during fasting and that it favors both mechanisms. In adipocytes, GDS signals through the Htr2b receptor to favor lipolysis by increasing phosphorylation and activity of hormone-sensitive lipase. In hepatocytes, GDS signaling through Htr2b promotes gluconeogenesis by enhancing activity of two rate-limiting gluconeogenic enzymes, FBPase and G6Pase. In addition, GDS signaling in hepatocytes prevents glucose uptake in a Glut2-dependent manner, thereby further favoring maintenance of blood glucose levels. As a result, inhibition of GDS synthesis can improve glucose intolerance caused by high-fat diet. Hence, GDS opposes deleterious consequences of food deprivation by favoring lipolysis and liver gluconeogenesis while preventing glucose uptake by hepatocytes. As a result, pharmacological inhibition of its synthesis may contribute to improve type 2 diabetes.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes.Clin Sci (Lond). 2015 Nov;129(10):839-50. doi: 10.1042/CS20150009. Epub 2015 Jul 13. Clin Sci (Lond). 2015. PMID: 26201094
-
Regulation of hepatic gluconeogenesis by nuclear factor Y transcription factor in mice.J Biol Chem. 2018 May 18;293(20):7894-7904. doi: 10.1074/jbc.RA117.000508. Epub 2018 Mar 12. J Biol Chem. 2018. PMID: 29530977 Free PMC article.
-
The nuclear bile acid receptor FXR is a PKA- and FOXA2-sensitive activator of fasting hepatic gluconeogenesis.J Hepatol. 2018 Nov;69(5):1099-1109. doi: 10.1016/j.jhep.2018.06.022. Epub 2018 Jul 5. J Hepatol. 2018. PMID: 29981427
-
Hepatic glucose uptake, gluconeogenesis and the regulation of glycogen synthesis.Diabetes Metab Res Rev. 2001 Jul-Aug;17(4):250-72. doi: 10.1002/dmrr.217. Diabetes Metab Res Rev. 2001. PMID: 11544610 Review.
-
Rationale and hurdles of inhibitors of hepatic gluconeogenesis in treatment of diabetes mellitus.Diabetes Res Clin Pract. 1995 Aug;28 Suppl:S195-200. doi: 10.1016/0168-8227(95)01079-s. Diabetes Res Clin Pract. 1995. PMID: 8529514 Review.
Cited by
-
The Regulatory Role of the Central and Peripheral Serotonin Network on Feeding Signals in Metabolic Diseases.Int J Mol Sci. 2022 Jan 29;23(3):1600. doi: 10.3390/ijms23031600. Int J Mol Sci. 2022. PMID: 35163521 Free PMC article. Review.
-
The Gut Microbiota Affects Host Pathophysiology as an Endocrine Organ: A Focus on Cardiovascular Disease.Nutrients. 2019 Dec 27;12(1):79. doi: 10.3390/nu12010079. Nutrients. 2019. PMID: 31892152 Free PMC article. Review.
-
The gut microbiome regulates host glucose homeostasis via peripheral serotonin.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):19802-19804. doi: 10.1073/pnas.1909311116. Epub 2019 Sep 16. Proc Natl Acad Sci U S A. 2019. PMID: 31527237 Free PMC article.
-
Selective inhibition of intestinal 5-HT improves neurobehavioral abnormalities caused by high-fat diet mice.Metab Brain Dis. 2019 Jun;34(3):747-761. doi: 10.1007/s11011-019-0392-x. Epub 2019 Apr 1. Metab Brain Dis. 2019. PMID: 30931486
-
Clostridium ramosum regulates enterochromaffin cell development and serotonin release.Sci Rep. 2019 Feb 4;9(1):1177. doi: 10.1038/s41598-018-38018-z. Sci Rep. 2019. PMID: 30718836 Free PMC article.
References
-
- Alegre M, Ciudad CJ, Fillat C, Guinovart JJ. Determination of glucose-6-phosphatase activity using the glucose dehydrogenase-coupled reaction. Anal Biochem. 1988;173:185–189. - PubMed
-
- Amireault P, Hatia S, Bayard E, Bernex F, Collet C, Callebert J, Launay JM, Hermine O, Schneider E, Mallet J, et al. Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13141–13146. - PMC - PubMed
-
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E, Holm C. Identification of novel phosphorylation sites in hormone-sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. The Journal of biological chemistry. 1998;273:215–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

