The PAADRN study: a design for a randomized controlled practical clinical trial to improve bone health
- PMID: 23085132
- PMCID: PMC3525745
- DOI: 10.1016/j.cct.2012.10.002
The PAADRN study: a design for a randomized controlled practical clinical trial to improve bone health
Abstract
Introduction: To describe the rationale and design of an NIH funded randomized controlled trial: the Patient Activation after DXA Result Notification (PAADRN) study. The aim of this trial is to evaluate the effect that a direct mailing of Dual-Energy X-ray Absorptiometry (DXA) results from bone density testing centers to patients will have on patients' knowledge, treatment and self-efficacy.
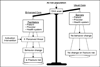
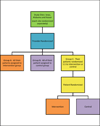
Methods: We will enroll approximately 7500 patients presenting for DXA at three study sites, the University of Iowa, the University of Alabama at Birmingham, and Kaiser Permanente of Atlanta, Georgia. We will randomize providers (and their respective patients) to either the intervention arm or usual care. Patients randomized to the intervention group will receive a letter with their DXA results and an educational brochure, while those randomized to usual care will receive their DXA results according to standard practice. The seven discrete outcomes are changes from baseline to 12-weeks and/or 52-weeks post-DXA in: (1) guideline concordant pharmacologic and non-pharmacologic therapy; (2) knowledge of DXA results; (3) osteoporosis-specific knowledge; (4) general health-related quality of life; (5) satisfaction with bone-related health care, (6) patient activation; and, (7) osteoporosis-specific self-efficacy.
Conclusion: This trial will offer evidence of the impact of a novel approach-direct-to-patient mailing of test results-to improve patient activation in their bone health care. The results will inform clinical practice for the communication of DXA and other test results.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures











Similar articles
-
Effects of a DXA result letter on satisfaction, quality of life, and osteoporosis knowledge: a randomized controlled trial.BMC Musculoskelet Disord. 2016 Aug 26;17(1):369. doi: 10.1186/s12891-016-1227-0. BMC Musculoskelet Disord. 2016. PMID: 27562713 Free PMC article. Clinical Trial.
-
The cost of a patient activation intervention for achieving successful outcomes: results from the PAADRN randomized controlled trial.Osteoporos Int. 2017 Oct;28(10):3061-3066. doi: 10.1007/s00198-017-4113-1. Epub 2017 Jun 15. Osteoporos Int. 2017. PMID: 28620779 Free PMC article. Clinical Trial.
-
The effects of a patient activation intervention on smoking and excessive drinking cessations: results from the PAADRN randomized controlled trial.Osteoporos Int. 2017 Oct;28(10):3055-3060. doi: 10.1007/s00198-017-4101-5. Epub 2017 Jun 1. Osteoporos Int. 2017. PMID: 28573377 Free PMC article. Clinical Trial.
-
Metasynthesis of Patient Attitudes Toward Bone Densitometry.J Gen Intern Med. 2018 Oct;33(10):1796-1804. doi: 10.1007/s11606-018-4587-3. Epub 2018 Jul 27. J Gen Intern Med. 2018. PMID: 30054881 Free PMC article.
-
How Can We Improve Osteoporosis Care? A Systematic Review and Meta-Analysis of the Efficacy of Quality Improvement Strategies for Osteoporosis.J Bone Miner Res. 2018 Sep;33(9):1585-1594. doi: 10.1002/jbmr.3437. Epub 2018 May 3. J Bone Miner Res. 2018. PMID: 29637658 Free PMC article.
Cited by
-
Pragmatic Clinical Trials in Osteoporosis.Curr Osteoporos Rep. 2019 Dec;17(6):521-526. doi: 10.1007/s11914-019-00551-9. Curr Osteoporos Rep. 2019. PMID: 31773402 Review.
-
Effects of a DXA result letter on satisfaction, quality of life, and osteoporosis knowledge: a randomized controlled trial.BMC Musculoskelet Disord. 2016 Aug 26;17(1):369. doi: 10.1186/s12891-016-1227-0. BMC Musculoskelet Disord. 2016. PMID: 27562713 Free PMC article. Clinical Trial.
-
Patient-reported reasons for nonadherence to recommended osteoporosis pharmacotherapy.J Am Pharm Assoc (2003). 2017 Jul-Aug;57(4):503-509. doi: 10.1016/j.japh.2017.05.003. Epub 2017 Jun 8. J Am Pharm Assoc (2003). 2017. PMID: 28602783 Free PMC article. Clinical Trial.
-
Patient Preferences for Test Result Notification.J Gen Intern Med. 2015 Nov;30(11):1651-6. doi: 10.1007/s11606-015-3344-0. J Gen Intern Med. 2015. PMID: 25944020 Free PMC article.
-
Validity, reliability, and responsiveness to change of the "Osteoporosis and You" knowledge scale.Osteoporos Int. 2017 Dec;28(12):3379-3388. doi: 10.1007/s00198-017-4204-z. Epub 2017 Sep 7. Osteoporos Int. 2017. PMID: 28879445 Free PMC article. Clinical Trial.
References
-
- National Osteoporosis, F. [cited 2012 February 3];Prevalence Report. 2011 Available from: http://www.nof.org/advocacy/resources/prevalencereport.
-
- U.S. Preventive Services Task Force. [cited 2012 February 3];Screening for Osteoporosis: Recommendation Statement. 2011 Available from: http://www.uspreventiveservicestaskforce.org/uspstf10/osteoporosis/osteo.... - PubMed
-
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet. 2002;359(9319):1761–1767. - PubMed
-
- Cram P, et al. Failure to recognize and act on abnormal test results: the case of screening bone densitometry. Jt Comm J Qual Patient Saf. 2005;31(2):90–97. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

