Post-translational modifications of myofilament proteins involved in length-dependent prolongation of relaxation in rabbit right ventricular myocardium
- PMID: 23085150
- PMCID: PMC3640662
- DOI: 10.1016/j.abb.2012.10.005
Post-translational modifications of myofilament proteins involved in length-dependent prolongation of relaxation in rabbit right ventricular myocardium
Abstract
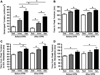
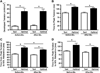
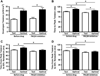
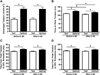
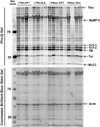
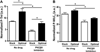
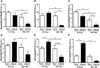
The phosphorylation state of several cardiac myofilament proteins changes with the level of stretch in intact, twitch-contracting cardiac muscles. It remains unclear which kinases are involved in the length-dependent phosphorylation of these proteins. We set out to investigate which kinases are involved after a step-wise change in cardiac muscle length. We hypothesize that myofilament protein phosphorylation by PKCβII and PKA alters contractile kinetics during length-dependent activation. Right ventricular intact trabeculae were isolated from New Zealand White rabbit hearts and stimulated to contract at 1Hz. Twitch force recordings where taken at taut and optimal muscle lengths before and after administration of kinase inhibitors at 37°C. PKCβII inhibition significantly decreased time from stimulation to peak force (TTP), time from peak force to 50% relaxation (RT50), and 90% relaxation (RT90) at optimal muscle length. This led to a loss in the length-dependent increase of RT50 and RT90 in the presence of the PKCβII inhibitor, whereas the length-dependent increase in RT50 and RT90 was seen in the controls. PKA inhibition using H-89 significantly decreased TTP at both taut and optimal muscle lengths. Detection of Ser/Thr phosphorylation with ProQ-diamond staining indicates a role for PKCβII in the phosphorylation of tropomyosin and myosin light chain-2 (MLC2) and PKA for tropomyosin, troponin-I, MLC2, myosin binding protein-C, troponin-T (TnT) 3 and TnT4. Our data provide evidence for two signaling kinases acting upon myofilament proteins during length-dependent activation, and provide further insight for length-dependent myofilament function.
Copyright © 2012. Published by Elsevier Inc.
Figures
Similar articles
-
Increased phosphorylation of tropomyosin, troponin I, and myosin light chain-2 after stretch in rabbit ventricular myocardium under physiological conditions.J Mol Cell Cardiol. 2010 May;48(5):1023-8. doi: 10.1016/j.yjmcc.2010.03.004. Epub 2010 Mar 16. J Mol Cell Cardiol. 2010. PMID: 20298699 Free PMC article.
-
Comprehensive assessment of chamber-specific and transmural heterogeneity in myofilament protein phosphorylation by top-down mass spectrometry.J Mol Cell Cardiol. 2015 Oct;87:102-12. doi: 10.1016/j.yjmcc.2015.08.007. Epub 2015 Aug 9. J Mol Cell Cardiol. 2015. PMID: 26268593 Free PMC article.
-
Interventricular differences in myofilament function in experimental congestive heart failure.Pflugers Arch. 2011 Dec;462(6):795-809. doi: 10.1007/s00424-011-1024-4. Epub 2011 Sep 17. Pflugers Arch. 2011. PMID: 21927813 Free PMC article.
-
The slow force response to stretch in atrial and ventricular myocardium from human heart: functional relevance and subcellular mechanisms.Prog Biophys Mol Biol. 2008 Jun-Jul;97(2-3):250-67. doi: 10.1016/j.pbiomolbio.2008.02.026. Epub 2008 Mar 14. Prog Biophys Mol Biol. 2008. PMID: 18466959 Free PMC article. Review.
-
Top-down mass spectrometry of cardiac myofilament proteins in health and disease.Proteomics Clin Appl. 2014 Aug;8(7-8):554-68. doi: 10.1002/prca.201400043. Proteomics Clin Appl. 2014. PMID: 24945106 Free PMC article. Review.
Cited by
-
Effect of hypothyroidism on contractile performance of isolated end-stage failing human myocardium.PLoS One. 2022 Apr 11;17(4):e0265731. doi: 10.1371/journal.pone.0265731. eCollection 2022. PLoS One. 2022. PMID: 35404981 Free PMC article.
-
Tri-modal regulation of cardiac muscle relaxation; intracellular calcium decline, thin filament deactivation, and cross-bridge cycling kinetics.Biophys Rev. 2014 Dec;6(3-4):273-289. doi: 10.1007/s12551-014-0143-5. Epub 2014 Jul 17. Biophys Rev. 2014. PMID: 28510030 Free PMC article.
-
Post-translational modification patterns on β-myosin heavy chain are altered in ischemic and nonischemic human hearts.Elife. 2022 May 3;11:e74919. doi: 10.7554/eLife.74919. Elife. 2022. PMID: 35502901 Free PMC article.
-
Length-Dependent Prolongation of Force Relaxation Is Unaltered by Delay of Intracellular Calcium Decline in Early-Stage Rabbit Right Ventricular Hypertrophy.Front Physiol. 2017 Dec 4;8:945. doi: 10.3389/fphys.2017.00945. eCollection 2017. Front Physiol. 2017. PMID: 29255420 Free PMC article.
-
Rabbit models of cardiac mechano-electric and mechano-mechanical coupling.Prog Biophys Mol Biol. 2016 Jul;121(2):110-22. doi: 10.1016/j.pbiomolbio.2016.05.003. Epub 2016 May 18. Prog Biophys Mol Biol. 2016. PMID: 27208698 Free PMC article. Review.
References
-
- Monasky MM, Varian KD, Davis JP, Janssen PM. Pflugers Arch. 2008;456:267–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

