Replication-dependent irreversible topoisomerase 1 poisoning is responsible for FdUMP[10] anti-leukemic activity
- PMID: 23085462
- PMCID: PMC3660094
- DOI: 10.1016/j.exphem.2012.10.007
Replication-dependent irreversible topoisomerase 1 poisoning is responsible for FdUMP[10] anti-leukemic activity
Abstract
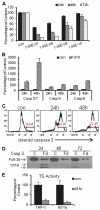
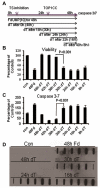
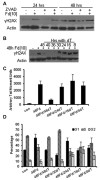
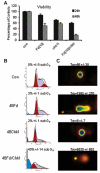
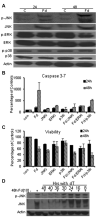
Previous studies have indicated that 5-Fluoro-2'-deoxyuridine-5'-O-monophosphate 10mer (FdUMP[10]) displays strong antileukemic activity through the dual targeting of thymidylate synthase (TS) and DNA topoisomerase 1 (Top1). The present studies were undertaken to clarify the relationship between the induction of a thymineless state and the formation of Top1 cleavage complexes (Top1CC) for inducing cell death and to clarify the role of DNA replication for induction of lethal DNA double-strand breaks (DSBs) in FdUMP[10]-treated acute myeloid leukemia (AML) cells. Human promyelocytic (HL60) and AML (KG1a, Molm13, THP-1) cells were synchronized by serum starvation and treated with FdUMP[10] with thymidine (Thy) rescue. Cells were assayed for TS inhibition, DNA DSBs, Top1CC, and apoptosis to clarify the interrelationship of TS inhibition and Top1CC for cell death. FdUMP[10] induced a thymineless state in AML cells and exogenous Thy administered within the first 18 hours of treatment rescued FdUMP[10]-induced Top1CC formation, γH2AX phosphorylation, and apoptosis induction. Exogenous Thy was not effective after cells had committed to mitosis and undergone cell division in the presence of FdUMP[10]. FdUMP[10] treatment resulted in Chk1 activation, and Chk1 inhibition enhanced FdUMP[10]-induced DNA damage and apoptosis. Jnk-signaling was required for FdUMP[10]-induced apoptosis in promyelocytic HL60 cells and in THP1 cells, but was antiapoptotic in Molm13 and to a lesser extent KG1a AML cells. The results are consistent with FdUMP[10] inducing a thymineless state, leading to misincorporation of FdU into genomic DNA of proliferating cells. Top1CC form in cells upon re-entry into S-phase, resulting in DNA double-strand breaks, and initiating apoptotic signaling that can be either muted or enhanced by Jnk-signaling depending on cell type.
Copyright © 2013 ISEH - Society for Hematology and Stem Cells. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
A novel polypyrimidine antitumor agent FdUMP[10] induces thymineless death with topoisomerase I-DNA complexes.Cancer Res. 2005 Jun 1;65(11):4844-51. doi: 10.1158/0008-5472.CAN-04-1302. Cancer Res. 2005. PMID: 15930305
-
Unique dual targeting of thymidylate synthase and topoisomerase1 by FdUMP[10] results in high efficacy against AML and low toxicity.Blood. 2012 Apr 12;119(15):3561-70. doi: 10.1182/blood-2011-06-362442. Epub 2012 Feb 23. Blood. 2012. PMID: 22362039 Free PMC article.
-
Mechanisms of action of FdUMP[10]: metabolite activation and thymidylate synthase inhibition.Oncol Rep. 2007 Jul;18(1):287-91. doi: 10.3892/or.18.1.287. Oncol Rep. 2007. PMID: 17549381
-
Apoptosis induced by DNA topoisomerase I and II inhibitors in human leukemic HL-60 cells.Leuk Lymphoma. 1994 Sep;15(1-2):21-32. doi: 10.3109/10428199409051674. Leuk Lymphoma. 1994. PMID: 7858500 Review.
-
Determinants of response to antimetabolites and their modulation by normal purine and pyrimidine metabolites.Cancer Treat Rep. 1981;65 Suppl 3:73-82. Cancer Treat Rep. 1981. PMID: 7049369 Review.
Cited by
-
Improved potency of F10 relative to 5-fluorouracil in colorectal cancer cells with p53 mutations.Cancer Drug Resist. 2018;1:48-58. doi: 10.20517/cdr.2018.01. Epub 2018 Mar 19. Cancer Drug Resist. 2018. PMID: 30613833 Free PMC article.
-
Thymineless Death by the Fluoropyrimidine Polymer F10 Involves Replication Fork Collapse and Is Enhanced by Chk1 Inhibition.Neoplasia. 2018 Dec;20(12):1236-1245. doi: 10.1016/j.neo.2018.10.006. Epub 2018 Nov 12. Neoplasia. 2018. PMID: 30439567 Free PMC article.
-
Entrapment of DNA topoisomerase-DNA complexes by nucleotide/nucleoside analogs.Cancer Drug Resist. 2019;2(4):994-1001. doi: 10.20517/cdr.2019.95. Epub 2019 Dec 19. Cancer Drug Resist. 2019. PMID: 31930190 Free PMC article.
-
F10 Inhibits Growth of PC3 Xenografts and Enhances the Effects of Radiation Therapy.J Clin Oncol Res. 2014 Jul-Aug;2(4):1028. J Clin Oncol Res. 2014. PMID: 26020060 Free PMC article.
-
AraC-FdUMP[10] Is a Next-Generation Fluoropyrimidine with Potent Antitumor Activity in PDAC and Synergy with PARG Inhibition.Mol Cancer Res. 2021 Apr;19(4):565-572. doi: 10.1158/1541-7786.MCR-20-0985. Epub 2021 Feb 16. Mol Cancer Res. 2021. PMID: 33593942 Free PMC article.
References
-
- Roboz GJ. Novel approaches to the treatment of acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2011;2011:43–50. - PubMed
-
- Buchner T, Berdel WE, Wormann B, et al. Treatment of older patients with AML. Crit Rev Oncol Hematol. 2005;56:247–259. - PubMed
-
- Robak T, Wierzbowska A. Current and emerging therapies for acute myeloid leukemia. Clin Ther. 2009;31:2349–2370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

