Redox regulation of stem/progenitor cells and bone marrow niche
- PMID: 23085514
- PMCID: PMC3637653
- DOI: 10.1016/j.freeradbiomed.2012.10.532
Redox regulation of stem/progenitor cells and bone marrow niche
Abstract
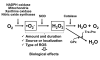
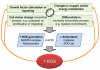
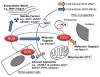
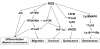
Bone marrow (BM)-derived stem and progenitor cell functions including self-renewal, differentiation, survival, migration, proliferation, and mobilization are regulated by unique cell-intrinsic and -extrinsic signals provided by their microenvironment, also termed the "niche." Reactive oxygen species (ROS), especially hydrogen peroxide (H(2)O(2)), play important roles in regulating stem and progenitor cell functions in various physiologic and pathologic responses. The low level of H(2)O(2) in quiescent hematopoietic stem cells (HSCs) contributes to maintaining their "stemness," whereas a higher level of H(2)O(2) within HSCs or their niche promotes differentiation, proliferation, migration, and survival of HSCs or stem/progenitor cells. Major sources of ROS are NADPH oxidase and mitochondria. In response to ischemic injury, ROS derived from NADPH oxidase are increased in the BM microenvironment, which is required for hypoxia and hypoxia-inducible factor-1α expression and expansion throughout the BM. This, in turn, promotes progenitor cell expansion and mobilization from BM, leading to reparative neovascularization and tissue repair. In pathophysiological states such as aging, atherosclerosis, heart failure, hypertension, and diabetes, excess amounts of ROS create an inflammatory and oxidative microenvironment, which induces cell damage and apoptosis of stem and progenitor cells. Understanding the molecular mechanisms of how ROS regulate the functions of stem and progenitor cells and their niche in physiological and pathological conditions will lead to the development of novel therapeutic strategies.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Rafii S, Avecilla S, Shmelkov S, Shido K, Tejada R, Moore MA, Heissig B, Hattori K. Angiogenic factors reconstitute hematopoiesis by recruiting stem cells from bone marrow microenvironment. Ann N Y Acad Sci. 2003;996:49–60. - PubMed
-
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. - PubMed
-
- Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

