Epithelial stem cells and implications for wound repair
- PMID: 23085626
- PMCID: PMC3518754
- DOI: 10.1016/j.semcdb.2012.10.001
Epithelial stem cells and implications for wound repair
Abstract
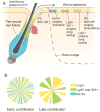
Activation of epithelial stem cells and efficient recruitment of their proliferating progeny plays a critical role in cutaneous wound healing. The reepithelialized wound epidermis has a mosaic composition consisting of progeny that can be traced back both to epidermal and several types of hair follicle stem cells. The contribution of hair follicle stem cells to wound epidermis is particularly intriguing as it involves lineage identity change from follicular to epidermal. Studies from our laboratory show that hair follicle-fated bulge stem cells commit only transient amplifying epidermal progeny that participate in the initial wound re-epithelialization, but eventually are outcompeted by other epidermal clones and largely disappear after a few months. Conversely, recently described stem cell populations residing in the isthmus portion of hair follicle contribute long-lasting progeny toward wound epidermis and, arguably, give rise to new interfollicular epidermal stem cells. The role of epithelial stem cells during wound healing is not limited to regenerating stratified epidermis. By studying regenerative response in large cutaneous wounds, our laboratory uncovered that epithelial cells in the center of the wound can acquire greater morphogenetic plasticity and, together with the underlying wound dermis, can engage in an embryonic-like process of hair follicle neogenesis. Future studies should uncover the cellular and signaling basis of this remarkable adult wound regeneration phenomenon.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures


References
-
- Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “Hair Growth-Wound Healing Connection”: Anagen Phase Promotes Wound Re-Epithelialization. J Investig Dermatol. 2011;131:518–28. - PubMed
-
- Brittan M, Braun KM, Reynolds LE, Conti FJ, Reynolds AR, Poulsom R, Alison MR, Wright NA, Hodivala-Dilke KM. Bone marrow cells engraft within the epidermis and proliferate in vivo with no evidence of cell fusion. J Pathol. 2005;205:1–13. - PubMed
-
- Cotsarelis G, Sun TT, Lavker RM. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell. 1990;61:1329– 37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

