Pharmacokinetics and pharmacodynamics of single and multiple doses of prasugrel in healthy native Chinese subjects
- PMID: 23085738
- PMCID: PMC4011359
- DOI: 10.1038/aps.2012.120
Pharmacokinetics and pharmacodynamics of single and multiple doses of prasugrel in healthy native Chinese subjects
Abstract
Aim: To characterize the pharmacokinetics (PKs), pharmacodynamics (PDs), and tolerability of different dose regimens of prasugrel in healthy Chinese subjects.
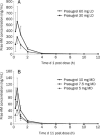
Methods: This was a single-centered, open-label, parallel-design study. Subjects received a single loading dose (LD) of prasugrel followed by once-daily maintenance dose (MD) for 10 d. They were enrolled into 3 groups: 60 mg LD/10 mg MD; 30 mg LD/7.5 mg MD; 30 mg LD/5 mg MD. Blood samples were collected after the first and last dose. The serum concentration of the active metabolite of prasugrel was determined using a LC/MS/MS method. Platelet aggregation was assessed using the VerifyNow-P2Y(12) assay.
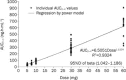
Results: Thirty-six healthy native Chinese subjects (19 males) aged 18-45 were enrolled; mean age and body weight were similar across the treatment groups (n=12 for each). The metabolite AUC(0-4) and C(max) increased dose-proportionally across the dose range of 5 mg to 60 mg. The median T(max) was 0.5 h in all groups. The PD parameters, indicated by the inhibition of ADP-induced platelet aggregation, were met more rapidly in the 60 mg group than the 30 mg group after the LD (94%-98%). This high degree of inhibition of platelet aggregation was maintained following the 10 mg MD (87%-90%) and was lower in the 7.5 mg and 5 mg MD groups (79%-83% and 64%-67%, respectively). Prasugrel was well tolerated in healthy Chinese subjects for single doses up to 60 mg and a MD of 10 mg for 10 d.
Conclusion: The PKs and PDs of the active metabolite of prasugrel were similar to those in Chinese subjects reported by a previous bridging study, which demonstrated that the exposure to the active metabolite in Chinese subjects was higher than in Caucasians.
Figures

Similar articles
-
The pharmacokinetics and pharmacodynamics of prasugrel in healthy Chinese, Japanese, and Korean subjects compared with healthy Caucasian subjects.Eur J Clin Pharmacol. 2010 Feb;66(2):127-35. doi: 10.1007/s00228-009-0737-1. Epub 2009 Nov 4. Eur J Clin Pharmacol. 2010. PMID: 19888568 Clinical Trial.
-
Pharmacodynamics and pharmacokinetics of single doses of prasugrel 30 mg and clopidogrel 300 mg in healthy Chinese and white volunteers: an open-label trial.Clin Ther. 2010 Feb;32(2):365-79. doi: 10.1016/j.clinthera.2010.02.015. Clin Ther. 2010. PMID: 20206794 Clinical Trial.
-
Effect of age on the pharmacokinetics and pharmacodynamics of prasugrel during multiple dosing: an open-label, single-sequence, clinical trial.Drugs Aging. 2009;26(9):781-90. doi: 10.2165/11315780-000000000-00000. Drugs Aging. 2009. PMID: 19728751 Clinical Trial.
-
Effect of intrinsic and extrinsic factors on the clinical pharmacokinetics and pharmacodynamics of prasugrel.Clin Pharmacokinet. 2010 Dec;49(12):777-98. doi: 10.2165/11537820-000000000-00000. Clin Pharmacokinet. 2010. PMID: 21053990 Review.
-
Pharmacokinetic basis of the antiplatelet action of prasugrel.Fundam Clin Pharmacol. 2012 Feb;26(1):39-46. doi: 10.1111/j.1472-8206.2011.00986.x. Epub 2011 Sep 5. Fundam Clin Pharmacol. 2012. PMID: 21895761 Review.
Cited by
-
Expert consensus document: World Heart Federation expert consensus statement on antiplatelet therapy in East Asian patients with ACS or undergoing PCI.Nat Rev Cardiol. 2014 Oct;11(10):597-606. doi: 10.1038/nrcardio.2014.104. Epub 2014 Aug 26. Nat Rev Cardiol. 2014. PMID: 25154978 Review.
-
Evaluation of Tolerability, Pharmacokinetics and Pharmacodynamics of Vicagrel, a Novel P2Y12 Antagonist, in Healthy Chinese Volunteers.Front Pharmacol. 2018 Jun 20;9:643. doi: 10.3389/fphar.2018.00643. eCollection 2018. Front Pharmacol. 2018. PMID: 29973877 Free PMC article.
-
Pharmacokinetics, mass balance, and metabolism of [14C]vicagrel, a novel irreversible P2Y12 inhibitor in humans.Acta Pharmacol Sin. 2021 Sep;42(9):1535-1546. doi: 10.1038/s41401-020-00547-7. Epub 2020 Nov 26. Acta Pharmacol Sin. 2021. PMID: 33244163 Free PMC article.
-
Pharmacometrics: a quantitative tool of pharmacological research.Acta Pharmacol Sin. 2012 Nov;33(11):1337-8. doi: 10.1038/aps.2012.149. Acta Pharmacol Sin. 2012. PMID: 23128515 Free PMC article. No abstract available.
-
Ticagrelor Versus Prasugrel in Acute Coronary Syndrome: Real-World Treatment and Safety.Medicines (Basel). 2025 May 14;12(2):13. doi: 10.3390/medicines12020013. Medicines (Basel). 2025. PMID: 40407605 Free PMC article.
References
-
- Baker WL, White CM. Role of prasugrel, a novel P2Y12 receptor antagonist, in the management of acute coronary syndromes. Am J Cardiovasc Drugs. 2009;9:213–29. - PubMed
-
- Niitsu Y, Jakubowski JA, Sugidachi A, Asai F. Pharmacology of CS-747 (prasugrel, LY640315), a novel, potent antiplatelet agent with in vivo P2Y12 receptor antagonist activity. Semin Thromb Hemost. 2005;31:184–94. - PubMed
-
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. - PubMed
-
- Ernest CS, II, Small DS, Rohatagi S, Salazar DE, Wallentin L, Winters KJ, et al. Population pharmacokinetics and pharamcodynamics of prasugrel and clopidogrel in aspirin-treated patients with stable coronary artery disease. J Pharmacokinet Pharmacodyn. 2008;35:593–618. - PubMed
-
- Small DS, Payne CD, Kothare P, Yuen E, Natanegara F, Teng Loh M, et al. Pharmacodynamics and pharmacokinetics of single doses of prasugrel 30 mg and clopidogrel 300 mg in healthy Chinese and white volunteers: an open-label trial. Clin Ther. 2010;32:365–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

