Simulation of the pharmacokinetics of bisoprolol in healthy adults and patients with impaired renal function using whole-body physiologically based pharmacokinetic modeling
- PMID: 23085739
- PMCID: PMC4011361
- DOI: 10.1038/aps.2012.103
Simulation of the pharmacokinetics of bisoprolol in healthy adults and patients with impaired renal function using whole-body physiologically based pharmacokinetic modeling
Abstract
Aim: To develop and evaluate a whole-body physiologically based pharmacokinetic (WB-PBPK) model of bisoprolol and to simulate its exposure and disposition in healthy adults and patients with renal function impairment.
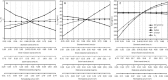
Methods: Bisoprolol dispositions in 14 tissue compartments were described by perfusion-limited compartments. Based the tissue composition equations and drug-specific properties such as log P, permeability, and plasma protein binding published in literatures, the absorption and whole-body distribution of bisoprolol was predicted using the 'Advanced Compartmental Absorption Transit' (ACAT) model and the whole-body disposition model, respectively. Renal and hepatic clearances were simulated using empirical scaling methods followed by incorporation into the WB-PBPK model. Model refinements were conducted after a comparison of the simulated concentration-time profiles and pharmacokinetic parameters with the observed data in healthy adults following intravenous and oral administration. Finally, the WB-PBPK model coupled with a Monte Carlo simulation was employed to predict the mean and variability of bisoprolol pharmacokinetics in virtual healthy subjects and patients.
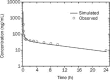
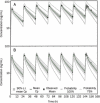
Results: The simulated and observed data after both intravenous and oral dosing showed good agreement for all of the dose levels in the reported normal adult population groups. The predicted pharmacokinetic parameters (AUC, C(max), and T(max)) were reasonably consistent (<1.3-fold error) with the observed values after single oral administration of doses ranging from of 5 to 20 mg using the refined WB-PBPK model. The simulated plasma profiles after multiple oral administration of bisoprolol in healthy adults and patient with renal impairment matched well with the observed profiles.
Conclusion: The WB-PBPK model successfully predicts the intravenous and oral pharmacokinetics of bisoprolol across multiple dose levels in diverse normal adult human populations and patients with renal insufficiency.
Figures





References
-
- Cruickshank JM. Beta-blockers and heart failure. Indian Heart J. 2010;62:101–10. - PubMed
-
- McGavin JK, Keating GM. Bisoprolol: a review of its use in chronic heart failure. Drugs. 2002;62:2677–96. - PubMed
-
- Papadopulos DP, Papademetriou V. Low-dose fixed combination of bisoprolol /hydrochlorothiazide as first line for hypertension: a review of the rationale and clinical evidence. Angiology. 2009;60:601–7. - PubMed
-
- CIBIS-II Investigators and Committees. The cardiac insufficiency bisoprolol study II (CIBIS-II): a randomised trial Lancet 19993539–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

