Association of CYP3A polymorphisms with the pharmacokinetics of cyclosporine A in early post-renal transplant recipients in China
- PMID: 23085740
- PMCID: PMC4001839
- DOI: 10.1038/aps.2012.136
Association of CYP3A polymorphisms with the pharmacokinetics of cyclosporine A in early post-renal transplant recipients in China
Abstract
Aim: To evaluate retrospectively the association of cytochrome P450 3A (CYP3A) and ATP-binding cassette sub-family B member 1 (ABCB1) gene polymorphisms with the pharmacokinetics of cyclosporine A (CsA) in Chinese renal transplant patients.
Methods: One hundred and twenty-six renal transplant patients were recruited. Blood samples were collected, and corresponding clinical indices were recorded on the seventh day after the procedure. The patients were genotyped for CYP3A4*1G, CYP3A5*3C, ABCB1 1236 C>T, ABCB1 2677 G>T/A, and ABCB1 3435 C>T polymorphisms. Whole blood trough concentrations of CsA at time zero (C(0)) were measured before the drug administration. A multiple regression model was developed to analyze the effects of genetic factors on the CsA dose-adjusted C(0) (C(0)/dose) based on several clinical indices.
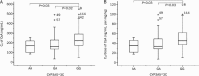
Results: The CYP3A5*3C polymorphism influenced the C(0) and C(0)/dose of CsA, which were significantly higher in patients with the GG genotype than in patients with the AA or GA genotypes. No significant differences were detected for other SNPs (CYP3A4*1G, ABCB1 1236 C>T, ABCB1 2677 G>T/A, and ABCB1 3435 C>T). In a univariate analysis using Pearson's correlation test, age, hemoglobin, blood urea nitrogen and blood creatinine levels were significantly correlated with the log-transformed CsA C(0)/dose. In the multiple regression model, CYP3A5*3C, age, hemoglobin and blood creatinine level were associated with the log-transformed CsA C(0)/dose.
Conclusion: CYP3A5*3C correlates with the C(0)/dose of CsA on the seventh day after renal transplantation. The allele is a putative indicator for the optimal CsA dosage in the early phase of renal transplantation in the Chinese population.
Figures
Similar articles
-
Polymorphisms in CYP3A5, CYP3A4, and ABCB1 are not associated with cyclosporine pharmacokinetics nor with cyclosporine clinical end points after renal transplantation.Ther Drug Monit. 2011 Apr;33(2):178-84. doi: 10.1097/FTD.0b013e31820feb8e. Ther Drug Monit. 2011. PMID: 21383650 Clinical Trial.
-
Influence of ABCB1, CYP3A4*18B and CYP3A5*3 polymorphisms on cyclosporine A pharmacokinetics in bone marrow transplant recipients.Pharmacol Rep. 2011;63(3):815-25. doi: 10.1016/s1734-1140(11)70594-1. Pharmacol Rep. 2011. PMID: 21857093
-
The effect of CYP3A4, CYP3A5 and MDR1 (ABCB1) single nucleotide polymorphisms on the pharmacokinetics of cyclosporine in Algerian stable renal transplant recipients.Mol Biol Rep. 2025 Jul 25;52(1):756. doi: 10.1007/s11033-025-10862-z. Mol Biol Rep. 2025. PMID: 40711648
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part I.Clin Pharmacokinet. 2010 Mar;49(3):141-75. doi: 10.2165/11317350-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20170205 Review.
-
Effect of CYP3A and ABCB1 single nucleotide polymorphisms on the pharmacokinetics and pharmacodynamics of calcineurin inhibitors: Part II.Clin Pharmacokinet. 2010 Apr;49(4):207-21. doi: 10.2165/11317550-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20214406 Review.
Cited by
-
The effects of the CYP3A5*3 variant on tacrolimus pharmacokinetics and outcomes in Tunisian kidney transplant recipients.Tunis Med. 2023 Oct 5;101(10):738-744. Tunis Med. 2023. PMID: 38465753 Free PMC article. English.
-
Clinical implementation of pharmacogenetics in kidney transplantation: calcineurin inhibitors in the starting blocks.Br J Clin Pharmacol. 2014 Apr;77(4):715-28. doi: 10.1111/bcp.12253. Br J Clin Pharmacol. 2014. PMID: 24118098 Free PMC article. Review.
-
Association between interleukin-18 promoter variants and tacrolimus pharmacokinetics in Chinese renal transplant patients.Eur J Clin Pharmacol. 2015 Feb;71(2):191-8. doi: 10.1007/s00228-014-1785-8. Epub 2014 Dec 10. Eur J Clin Pharmacol. 2015. PMID: 25487141 Clinical Trial.
-
Maternal medication use, fetal 3435 C>T polymorphism of the ABCB1 gene, and risk of isolated septal defects in a Han Chinese population.Pediatr Cardiol. 2014 Oct;35(7):1132-41. doi: 10.1007/s00246-014-0906-6. Epub 2014 Apr 17. Pediatr Cardiol. 2014. PMID: 24740628
-
Pharmacogenomics and personalized medicine: a review focused on their application in the Chinese population.Acta Pharmacol Sin. 2015 May;36(5):535-43. doi: 10.1038/aps.2015.10. Epub 2015 Apr 20. Acta Pharmacol Sin. 2015. PMID: 25891088 Free PMC article. Review.
References
-
- Colombo D, Ammirati E. Cyclosporine in transplantation – a history of converging timelines. J Biol Regul Homeost Agents. 2011;25:493–504. - PubMed
-
- Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184–98. - PubMed
-
- Garcia-Saiz M, Lopez-Gil A, Alfonso I, Boada JN, Armijo JA. Factors influencing cyclosporine blood concentration-dose ratio. Ann Pharmacother. 2002;36:193–9. - PubMed
-
- Roy JN, Lajoie J, Zijenah LS, Barama A, Poirier C, Ward BJ, et al. CYP3A5 genetic polymorphisms in different ethnic populations. Drug Metab Dispos. 2005;33:884–7. - PubMed
-
- Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical