NANOG modulates stemness in human colorectal cancer
- PMID: 23085761
- PMCID: PMC3556342
- DOI: 10.1038/onc.2012.461
NANOG modulates stemness in human colorectal cancer
Abstract
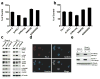
NANOG is a stem cell transcription factor that is essential for embryonic development, reprogramming normal adult cells and malignant transformation and progression. The nearly identical retrogene NANOGP8 is expressed in multiple cancers, but generally not in normal tissues and its function is not well defined. Our postulate is that NANOGP8 directly modulates the stemness of individual human colorectal carcinoma (CRC) cells. Stemness was measured in vitro as the spherogenicity of single CRC cells in serum-free medium and the size of the side population (SP) and in vivo as tumorigenicity and experimental metastatic potential in NOD/SCID mice. We found that 80% of clinical liver metastases express a NANOG with 75% of the positive metastases containing NANOGP8 transcripts. In all, 3-62% of single cells within six CRC lines form spheroids in serum-free medium in suspension. NANOGP8 is translated into protein. The relative expression of a NANOG gene increased 8- to 122-fold during spheroid formation, more than the increase in OCT4 or SOX2 transcripts with NANOGP8 the more prevalent family member. Short hairpin RNA (shRNA) to NANOG not only inhibits spherogenicity but also reduces expression of OCT4 and SOX2, the size of the SP and tumor growth in vivo. Inhibition of NANOG gene expression is associated with inhibition of proliferation and decreased phosphorylation of G2-related cell-cycle proteins. Overexpression of NANOGP8 rescues single-cell spherogenicity when NANOG gene expression is inhibited and increases the SP in CRC. Thus, NANOGP8 can substitute for NANOG in directly promoting stemness in CRC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

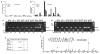
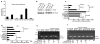


Similar articles
-
LGR5 Is a Gastric Cancer Stem Cell Marker Associated with Stemness and the EMT Signature Genes NANOG, NANOGP8, PRRX1, TWIST1, and BMI1.PLoS One. 2016 Dec 29;11(12):e0168904. doi: 10.1371/journal.pone.0168904. eCollection 2016. PLoS One. 2016. PMID: 28033430 Free PMC article.
-
Inhibition of NANOG/NANOGP8 downregulates MCL-1 in colorectal cancer cells and enhances the therapeutic efficacy of BH3 mimetics.Clin Cancer Res. 2014 Nov 1;20(21):5446-55. doi: 10.1158/1078-0432.CCR-14-1134. Epub 2014 Sep 10. Clin Cancer Res. 2014. PMID: 25208882 Free PMC article.
-
Embryonic NANOG activity defines colorectal cancer stem cells and modulates through AP1- and TCF-dependent mechanisms.Stem Cells. 2012 Oct;30(10):2076-87. doi: 10.1002/stem.1182. Stem Cells. 2012. PMID: 22851508
-
Regulation of NANOG in cancer cells.Mol Carcinog. 2015 Sep;54(9):679-87. doi: 10.1002/mc.22340. Epub 2015 May 27. Mol Carcinog. 2015. PMID: 26013997 Free PMC article. Review.
-
The oncogenic potential of NANOG: An important cancer induction mediator.J Cell Physiol. 2021 Apr;236(4):2443-2458. doi: 10.1002/jcp.30063. Epub 2020 Sep 22. J Cell Physiol. 2021. PMID: 32960465 Review.
Cited by
-
Molecular targets and therapeutics in chemoresistance of triple-negative breast cancer.Med Oncol. 2021 Nov 23;39(1):14. doi: 10.1007/s12032-021-01610-x. Med Oncol. 2021. PMID: 34812991 Review.
-
Soft fibrin matrix downregulates DAB2IP to promote Nanog-dependent growth of colon tumor-repopulating cells.Cell Death Dis. 2019 Feb 15;10(3):151. doi: 10.1038/s41419-019-1309-7. Cell Death Dis. 2019. PMID: 30770783 Free PMC article.
-
NANOG reprograms prostate cancer cells to castration resistance via dynamically repressing and engaging the AR/FOXA1 signaling axis.Cell Discov. 2016 Nov 15;2:16041. doi: 10.1038/celldisc.2016.41. eCollection 2016. Cell Discov. 2016. PMID: 27867534 Free PMC article.
-
Insights into the Nanog gene: A propeller for stemness in primitive stem cells.Int J Biol Sci. 2016 Oct 25;12(11):1372-1381. doi: 10.7150/ijbs.16349. eCollection 2016. Int J Biol Sci. 2016. PMID: 27877089 Free PMC article. Review.
-
In vivo epigenetic reprogramming of primary human colon cancer cells enhances metastases.J Mol Cell Biol. 2016 Apr;8(2):157-73. doi: 10.1093/jmcb/mjv034. Epub 2015 Jun 1. J Mol Cell Biol. 2016. PMID: 26031752 Free PMC article.
References
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. - PubMed
-
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

