LCAA, a novel factor required for magnesium protoporphyrin monomethylester cyclase accumulation and feedback control of aminolevulinic acid biosynthesis in tobacco
- PMID: 23085838
- PMCID: PMC3510121
- DOI: 10.1104/pp.112.206045
LCAA, a novel factor required for magnesium protoporphyrin monomethylester cyclase accumulation and feedback control of aminolevulinic acid biosynthesis in tobacco
Abstract
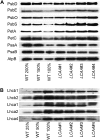
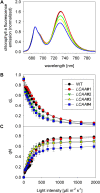
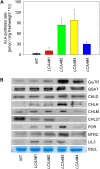
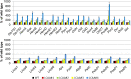
Low Chlorophyll Accumulation A (LCAA) antisense plants were obtained from a screen for genes whose partial down-regulation results in a strong chlorophyll deficiency in tobacco (Nicotiana tabacum). The LCAA mutants are affected in a plastid-localized protein of unknown function, which is conserved in cyanobacteria and all photosynthetic eukaryotes. They suffer from drastically reduced light-harvesting complex (LHC) contents, while the accumulation of all other photosynthetic complexes per leaf area is less affected. As the disturbed accumulation of LHC proteins could be either attributable to a defect in LHC biogenesis itself or to a bottleneck in chlorophyll biosynthesis, chlorophyll synthesis rates and chlorophyll synthesis intermediates were measured. LCAA antisense plants accumulate magnesium (Mg) protoporphyrin monomethylester and contain reduced protochlorophyllide levels and a reduced content of CHL27, a subunit of the Mg protoporphyrin monomethylester cyclase. Bimolecular fluorescence complementation assays confirm a direct interaction between LCAA and CHL27. 5-Aminolevulinic acid synthesis rates are increased and correlate with an increased content of glutamyl-transfer RNA reductase. We suggest that LCAA encodes an additional subunit of the Mg protoporphyrin monomethylester cyclase, is required for the stability of CHL27, and contributes to feedback-control of 5-aminolevulinic acid biosynthesis, the rate-limiting step of chlorophyll biosynthesis.
Figures



Similar articles
-
The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice.Plant Mol Biol. 2016 Sep;92(1-2):177-91. doi: 10.1007/s11103-016-0513-4. Epub 2016 Aug 11. Plant Mol Biol. 2016. PMID: 27514852
-
A novel tetratricopeptide-repeat protein, TTP1, forms complexes with glutamyl-tRNA reductase and protochlorophyllide oxidoreductase during tetrapyrrole biosynthesis.J Exp Bot. 2024 Mar 27;75(7):2027-2045. doi: 10.1093/jxb/erad491. J Exp Bot. 2024. PMID: 38070484 Free PMC article.
-
Potential roles of YCF54 and ferredoxin-NADPH reductase for magnesium protoporphyrin monomethylester cyclase.Plant J. 2018 May;94(3):485-496. doi: 10.1111/tpj.13869. Epub 2018 Mar 22. Plant J. 2018. PMID: 29443418
-
The regulation of enzymes involved in chlorophyll biosynthesis.Eur J Biochem. 1996 Apr 15;237(2):323-43. doi: 10.1111/j.1432-1033.1996.00323.x. Eur J Biochem. 1996. PMID: 8647070 Review.
-
Biosynthesis of chlorophylls from protoporphyrin IX.Nat Prod Rep. 2003 Jun;20(3):327-41. doi: 10.1039/b110549n. Nat Prod Rep. 2003. PMID: 12828371 Review.
Cited by
-
The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice.Plant Mol Biol. 2016 Sep;92(1-2):177-91. doi: 10.1007/s11103-016-0513-4. Epub 2016 Aug 11. Plant Mol Biol. 2016. PMID: 27514852
-
Evolutionary Aspects and Regulation of Tetrapyrrole Biosynthesis in Cyanobacteria under Aerobic and Anaerobic Environments.Life (Basel). 2015 Mar 30;5(2):1172-203. doi: 10.3390/life5021172. Life (Basel). 2015. PMID: 25830590 Free PMC article. Review.
-
RAPTOR Controls Developmental Growth Transitions by Altering the Hormonal and Metabolic Balance.Plant Physiol. 2018 Jun;177(2):565-593. doi: 10.1104/pp.17.01711. Epub 2018 Apr 23. Plant Physiol. 2018. PMID: 29686055 Free PMC article.
-
Sulfite Reductase Co-suppression in Tobacco Reveals Detoxification Mechanisms and Downstream Responses Comparable to Sulfate Starvation.Front Plant Sci. 2018 Oct 15;9:1423. doi: 10.3389/fpls.2018.01423. eCollection 2018. Front Plant Sci. 2018. PMID: 30374361 Free PMC article.
-
Mutation in Mg-Protoporphyrin IX Monomethyl Ester Cyclase Decreases Photosynthesis Capacity in Rice.PLoS One. 2017 Jan 27;12(1):e0171118. doi: 10.1371/journal.pone.0171118. eCollection 2017. PLoS One. 2017. PMID: 28129387 Free PMC article.
References
-
- Andersson J, Wentworth M, Walters RG, Howard CA, Ruban AV, Horton P, Jansson S. (2003) Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of photosystem II: effects on photosynthesis, grana stacking and fitness. Plant J 35: 350–361 - PubMed
-
- Arsova B, Hoja U, Wimmelbacher M, Greiner E, Ustün S, Melzer M, Petersen K, Lein W, Börnke F. (2010) Plastidial thioredoxin z interacts with two fructokinase-like proteins in a thiol-dependent manner: evidence for an essential role in chloroplast development in Arabidopsis and Nicotiana benthamiana. Plant Cell 22: 1498–1515 - PMC - PubMed
-
- Bannai H, Tamada Y, Maruyama O, Nakai K, Miyano S. (2002) Extensive feature detection of N-terminal protein sorting signals. Bioinformatics 18: 298–305 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

