VEGF and Notch in tip and stalk cell selection
- PMID: 23085847
- PMCID: PMC3530037
- DOI: 10.1101/cshperspect.a006569
VEGF and Notch in tip and stalk cell selection
Abstract
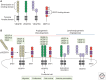
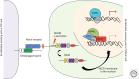
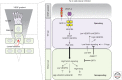
Sprouting angiogenesis is a dynamic process in which endothelial cells collectively migrate, shape new lumenized tubes, make new connections, and remodel the nascent network into a hierarchically branched and functionally perfused vascular bed. Endothelial cells in the nascent sprout adopt two distinct cellular phenotypes--known as tip and stalk cells--with specialized functions and gene expression patterns. VEGF and Notch signaling engage in an intricate cross talk to balance tip and stalk cell formation and to regulate directed tip cell migration and stalk cell proliferation. In this article, we summarize the current knowledge and implications of the tip/stalk cell concepts and the quantitative and dynamic integration of VEGF and Notch signaling in tip and stalk cell selection.
Figures



References
-
- Autiero M, Waltenberger J, Communi D, Kranz A, Moons L, Lambrechts D, Kroll J, Plaisance S, De Mol M, Bono F, et al. 2003. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat Med 9: 936–943 - PubMed
-
- Barleon B, Sozzani S, Zhou D, Weich HA, Mantovani A, Marme D 1996. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87: 3336–3343 - PubMed
-
- Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F 2010. VEGFR2 (KDR/Flk1) signalling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron 66: 205–219 - PubMed
-
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH 2009. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137: 1124–1135 - PubMed
-
- Bentley K, Gerhardt H, Bates PA 2008. Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol 250: 25–36 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
