The FUS about arginine methylation in ALS and FTLD
- PMID: 23085990
- PMCID: PMC3501219
- DOI: 10.1038/emboj.2012.291
The FUS about arginine methylation in ALS and FTLD
Abstract
EMBO J (2012) 31 22, 4258–4275 doi:; DOI: 10.1038/emboj.2012.261; published online September 11 2012
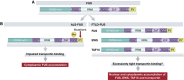
In a time where links between amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) neurodegeneration are becoming increasingly clear, it is important to establish the convergent and divergent mechanisms responsible for this. Accordingly, Dormann et al (2012) have identified that methylation of the Fused in sarcoma (FUS) RGG3 domain is involved in the cytoplasmic mislocalisation of ALS-FUS mutants, through a transportin-dependent mechanism. By contrast, hypomethylation in this domain may play a role in the aberrant accumulation of FUS in FTLD-FUS. This work showcases arginine methylation as a phenomenon to watch out for in neurodegenerative pathology.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Comment on
-
Arginine methylation next to the PY-NLS modulates Transportin binding and nuclear import of FUS.EMBO J. 2012 Nov 14;31(22):4258-75. doi: 10.1038/emboj.2012.261. Epub 2012 Sep 11. EMBO J. 2012. PMID: 22968170 Free PMC article.
References
-
- Araya N, Hiraga H, Kako K, Arao Y, Kato S, Fukamizu A (2005) Transcriptional down-regulation through nuclear exclusion of EWS methylated by PRMT1. Biochem Biophys Res Commun 329: 653–660 - PubMed
-
- Hung C-J, Lee Y-J, Chen D-H, Li C (2009) Proteomic analysis of methylarginine-containing proteins in HeLa cells by two-dimensional gel electrophoresis and immunoblotting with a methylarginine-specific antibody. Protein J 28: 139–147 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

