LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria
- PMID: 23086228
- PMCID: PMC3533418
- DOI: 10.1124/jpet.112.199216
LCL124, a cationic analog of ceramide, selectively induces pancreatic cancer cell death by accumulating in mitochondria
Abstract
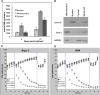
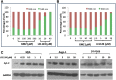
Treatment of pancreatic cancer that cannot be surgically resected currently relies on minimally beneficial cytotoxic chemotherapy with gemcitabine. As the fourth leading cause of cancer-related death in the United States with dismal survival statistics, pancreatic cancer demands new and more effective treatment approaches. Resistance to gemcitabine is nearly universal and appears to involve defects in the intrinsic/mitochondrial apoptotic pathway. The bioactive sphingolipid ceramide is a critical mediator of apoptosis initiated by a number of therapeutic modalities. It is noteworthy that insufficient ceramide accumulation has been linked to gemcitabine resistance in multiple cancer types, including pancreatic cancer. Taking advantage of the fact that cancer cells frequently have more negatively charged mitochondria, we investigated a means to circumvent resistance to gemcitabine by targeting delivery of a cationic ceramide (l-t-C6-CCPS [LCL124: ((2S,3S,4E)-2-N-[6'-(1″-pyridinium)-hexanoyl-sphingosine bromide)]) to cancer cell mitochondria. LCL124 was effective in initiating apoptosis by causing mitochondrial depolarization in pancreatic cancer cells but demonstrated significantly less activity against nonmalignant pancreatic ductal epithelial cells. Furthermore, we demonstrate that the mitochondrial membrane potentials of the cancer cells were more negative than nonmalignant cells and that dissipation of this potential abrogated cell killing by LCL124, establishing that the effectiveness of this compound is potential-dependent. LCL124 selectively accumulated in and inhibited the growth of xenografts in vivo, confirming the tumor selectivity and therapeutic potential of cationic ceramides in pancreatic cancer. It is noteworthy that gemcitabine-resistant pancreatic cancer cells became more sensitive to subsequent treatment with LCL124, suggesting that this compound may be a uniquely suited to overcome gemcitabine resistance in pancreatic cancer.
Figures




References
-
- Aronov O, Horowitz AT, Gabizon A, Fuertes MA, Pérez JM, Gibson D. (2004) Nuclear localization signal-targeted poly(ethylene glycol) conjugates as potential carriers and nuclear localizing agents for carboplatin analogues. Bioconjug Chem 15:814–823 - PubMed
-
- Arora AS, Jones BJ, Patel TC, Bronk SF, Gores GJ. (1997) Ceramide induces hepatocyte cell death through disruption of mitochondrial function in the rat. Hepatology 25:958–963 - PubMed
-
- Bai J, Sui J, Demirjian A, Vollmer CM, Jr, Marasco W, Callery MP. (2005) Predominant Bcl-XL knockdown disables antiapoptotic mechanisms: tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res 65:2344–2352 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

