Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling
- PMID: 23086611
- PMCID: PMC3552813
- DOI: 10.1289/ehp.1205374
Heavy metal lead exposure, osteoporotic-like phenotype in an animal model, and depression of Wnt signaling
Erratum in
- Environ Health Perspect. 2013 Feb;121(2):a43
Abstract
Background: Exposure to lead (Pb) from environmental and industrial sources remains an overlooked serious public health risk. Elucidating the effect of Pb on bone cell function is therefore critical for understanding its risk associated with diseases of low bone mass.
Objectives: We tested the hypothesis that Pb negatively affects bone mass. We also assessed the underlying mechanisms of Pb on bone signaling pathways.
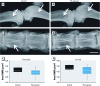
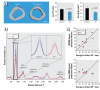
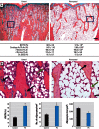
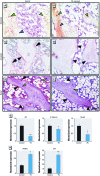
Methods: We used a model of low-level Pb exposure in a rodent beginning before conception and continuing over 18 months. We characterized the effect of Pb on bone quality using dual-energy X-ray absorptiometry (DXA), micro-computed tomography, Raman spectroscopy, and histology. We assessed the effect of Pb on bone and adipocyte formation by mineral deposition, lipid droplet formation, and Western blot and RNA analysis.
Results: Pb-exposed animals had decreased bone mass that resulted in bones that were more susceptible to fracture. Pb decreased osteoblastic cell number leading to a depression of bone formation. Accompanying this, Pb exposure elevated sclerostin protein levels in the skeleton, and correspondingly reduced levels of β-catenin and Runx2 in stromal precursor cells. Pb also increased skeletal expression of peroxisome proliferator-activated receptor-γ (PPAR-γ). These results indicate a shift in mesenchymal differentiation wherein Pb promoted enhanced adipogenesis and decreased osteoblastogenesis. Substantial differences in bone marrow composition were observed, highlighted by an increase in adipocytes.
Conclusions: The disruption Pb has on bone mass and bone homeostasis is principally explained by inhibition of the Wnt/β-catenin pathway, which may provide a molecular basis for novel therapeutic strategies to combat Pb-induced bone pathologies.
Conflict of interest statement
The authors declare they have no actual or potential competing financial interests.
Figures

References
-
- Bagchi D, Preuss HG. Effects of acute and chronic oval exposure of lead on blood pressure and bone mineral density in rats. J Inorg Biochem. 2005;99(5):1155–1164. - PubMed
-
- Barth RW, Williams JL, Kaplan FS. Osteon morphometry in females with femoral neck fractures. Clin Orthop Relat Res. 1992;283:178–186. - PubMed
-
- Carmouche JJ, Puzas JE, Zhang X, Tiyapatanaputi P, Cory-Slechta DA, Gelein R, et al. Lead exposure inhibits fracture healing and is associated with increased chondrogenesis, delay in cartilage mineralization, and a decrease in osteoprogenitor frequency. Environ Health Perspect. 2005;113:749–755. - PMC - PubMed
-
- Centers for Disease Control and Prevention. Preventing Lead Poisoning in Young Children. Atlanta, GA:Centers for Disease Control and Prevention. 1991. Available: http://www.cdc.gov/nceh/lead/publications/prevleadpoisoning.pdf [accessed 27 November 2012]
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

