Bacteria differentially induce degradation of Bcl-xL, a survival protein, by human platelets
- PMID: 23086749
- PMCID: PMC3525025
- DOI: 10.1182/blood-2012-04-420661
Bacteria differentially induce degradation of Bcl-xL, a survival protein, by human platelets
Abstract
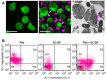
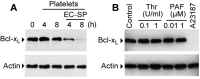
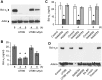
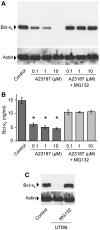
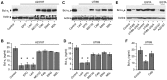
Bacteria can enter the bloodstream in response to infectious insults. Bacteremia elicits several immune and clinical complications, including thrombocytopenia. A primary cause of thrombocytopenia is shortened survival of platelets. We demonstrate that pathogenic bacteria induce apoptotic events in platelets that include calpain-mediated degradation of Bcl-x(L), an essential regulator of platelet survival. Specifically, bloodstream bacterial isolates from patients with sepsis induce lateral condensation of actin, impair mitochondrial membrane potential, and degrade Bcl-x(L) protein in platelets. Bcl-x(L) protein degradation is enhanced when platelets are exposed to pathogenic Escherichia coli that produce the pore-forming toxin α-hemolysin, a response that is markedly attenuated when the gene is deleted from E coli. We also found that nonpathogenic E coli gain degrading activity when they are forced to express α-hemolysin. Like α-hemolysin, purified α-toxin readily degrades Bcl-x(L) protein in platelets, as do clinical Staphylococcus aureus isolates that produce α-toxin. Inhibition of calpain activity, but not the proteasome, rescues Bcl-x(L) protein degradation in platelets coincubated with pathogenic E coli including α-hemolysin producing strains. This is the first evidence that pathogenic bacteria can trigger activation of the platelet intrinsic apoptosis program and our results suggest a new mechanism by which bacterial pathogens might cause thrombocytopenia in patients with bloodstream infections.
Figures
Similar articles
-
Essential roles for platelets during neutrophil-dependent or lymphocyte-mediated defense against bacterial pathogens.Blood Coagul Fibrinolysis. 2016 Sep;27(6):667-72. doi: 10.1097/MBC.0000000000000455. Blood Coagul Fibrinolysis. 2016. PMID: 26588444
-
A novel conditional platelet depletion mouse model reveals the importance of platelets in protection against Staphylococcus aureus bacteremia.J Thromb Haemost. 2015 Feb;13(2):303-13. doi: 10.1111/jth.12795. Epub 2014 Dec 30. J Thromb Haemost. 2015. PMID: 25418277 Free PMC article.
-
Staphylococcus aureus α-toxin triggers the synthesis of B-cell lymphoma 3 by human platelets.Toxins (Basel). 2011 Feb;3(2):120-33. doi: 10.3390/toxins3020120. Epub 2011 Jan 28. Toxins (Basel). 2011. PMID: 22069700 Free PMC article.
-
Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases.Int J Mol Sci. 2020 Oct 14;21(20):7591. doi: 10.3390/ijms21207591. Int J Mol Sci. 2020. PMID: 33066573 Free PMC article. Review.
-
Platelet intrinsic apoptosis.Thromb Res. 2023 Nov;231:206-213. doi: 10.1016/j.thromres.2022.11.024. Epub 2022 Dec 12. Thromb Res. 2023. PMID: 36739256 Review.
Cited by
-
Role of Extracellular Trap Release During Bacterial and Viral Infection.Front Microbiol. 2022 Jan 26;13:798853. doi: 10.3389/fmicb.2022.798853. eCollection 2022. Front Microbiol. 2022. PMID: 35154050 Free PMC article. Review.
-
Elevated angiotensin II induces platelet apoptosis through promoting oxidative stress in an AT1R-dependent manner during sepsis.J Cell Mol Med. 2021 Apr;25(8):4124-4135. doi: 10.1111/jcmm.16382. Epub 2021 Feb 23. J Cell Mol Med. 2021. PMID: 33624364 Free PMC article.
-
Crosstalk Between Platelets and Microbial Pathogens.Front Immunol. 2020 Aug 7;11:1962. doi: 10.3389/fimmu.2020.01962. eCollection 2020. Front Immunol. 2020. PMID: 32849656 Free PMC article. Review.
-
Anticancer compound ABT-263 accelerates apoptosis in virus-infected cells and imbalances cytokine production and lowers survival rates of infected mice.Cell Death Dis. 2013 Jul 25;4(7):e742. doi: 10.1038/cddis.2013.267. Cell Death Dis. 2013. PMID: 23887633 Free PMC article.
-
The Immune Nature of Platelets Revisited.Transfus Med Rev. 2020 Oct;34(4):209-220. doi: 10.1016/j.tmrv.2020.09.005. Epub 2020 Sep 19. Transfus Med Rev. 2020. PMID: 33051111 Free PMC article. Review.
References
-
- Shorr AF, Tabak YP, Killian AD, Gupta V, Liu LZ, Kollef MH. Healthcare-associated bloodstream infection: a distinct entity? Insights from a large U.S. database. Crit Care Med. 2006;34(10):2588–2595. - PubMed
-
- Weber DJ, Rutala WA. Central line-associated bloodstream infections: prevention and management. Infect Dis Clin North Am. 2011;25(1):77–102. - PubMed
-
- Niven DJ, Fick GH, Kirkpatrick AW, Grant V, Laupland KB. Cost and outcomes of nosocomial bloodstream infections complicating major traumatic injury. J Hosp Infect. 2010;76(4):296–299. - PubMed
-
- Petti CA, Sanders LL, Trivette SL, Briggs J, Sexton DJ. Postoperative bacteremia secondary to surgical site infection. Clin Infect Dis. 2002;34(3):305–308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL44525/HL/NHLBI NIH HHS/United States
- R03 AG040631/AG/NIA NIH HHS/United States
- R01 AI095647/AI/NIAID NIH HHS/United States
- HL66277/HL/NHLBI NIH HHS/United States
- R01 HL091754/HL/NHLBI NIH HHS/United States
- K23 HL092161/HL/NHLBI NIH HHS/United States
- R37 HL044525/HL/NHLBI NIH HHS/United States
- AI090369/AI/NIAID NIH HHS/United States
- R01 HL044525/HL/NHLBI NIH HHS/United States
- AI088086/AI/NIAID NIH HHS/United States
- R21 AI090369/AI/NIAID NIH HHS/United States
- HL91754/HL/NHLBI NIH HHS/United States
- UL1 TR001067/TR/NCATS NIH HHS/United States
- R01 HL066277/HL/NHLBI NIH HHS/United States
- U54HL112311/HL/NHLBI NIH HHS/United States
- 5T32DK007115-35/DK/NIDDK NIH HHS/United States
- R01 HL090870/HL/NHLBI NIH HHS/United States
- AI095647/AI/NIAID NIH HHS/United States
- K23 HL92161/HL/NHLBI NIH HHS/United States
- U54 HL112311/HL/NHLBI NIH HHS/United States
- T32 DK007115/DK/NIDDK NIH HHS/United States
- R21 AI088086/AI/NIAID NIH HHS/United States
- MOP-81208/CAPMC/ CIHR/Canada
- HL90870/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

