Human mismatch repair protein hMutLα is required to repair short slipped-DNAs of trinucleotide repeats
- PMID: 23086927
- PMCID: PMC3516732
- DOI: 10.1074/jbc.M112.420398
Human mismatch repair protein hMutLα is required to repair short slipped-DNAs of trinucleotide repeats
Abstract
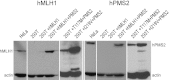
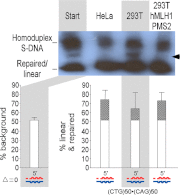
Mismatch repair (MMR) is required for proper maintenance of the genome by protecting against mutations. The mismatch repair system has also been implicated as a driver of certain mutations, including disease-associated trinucleotide repeat instability. We recently revealed a requirement of hMutSβ in the repair of short slip-outs containing a single CTG repeat unit (1). The involvement of other MMR proteins in short trinucleotide repeat slip-out repair is unknown. Here we show that hMutLα is required for the highly efficient in vitro repair of single CTG repeat slip-outs, to the same degree as hMutSβ. HEK293T cell extracts, deficient in hMLH1, are unable to process single-repeat slip-outs, but are functional when complemented with hMutLα. The MMR-deficient hMLH1 mutant, T117M, which has a point mutation proximal to the ATP-binding domain, is defective in slip-out repair, further supporting a requirement for hMLH1 in the processing of short slip-outs and possibly the involvement of hMHL1 ATPase activity. Extracts of hPMS2-deficient HEC-1-A cells, which express hMLH1, hMLH3, and hPMS1, are only functional when complemented with hMutLα, indicating that neither hMutLβ nor hMutLγ is sufficient to repair short slip-outs. The resolution of clustered short slip-outs, which are poorly repaired, was partially dependent upon a functional hMutLα. The joint involvement of hMutSβ and hMutLα suggests that repeat instability may be the result of aberrant outcomes of repair attempts.
Figures


Similar articles
-
Isolated short CTG/CAG DNA slip-outs are repaired efficiently by hMutSbeta, but clustered slip-outs are poorly repaired.Proc Natl Acad Sci U S A. 2010 Jul 13;107(28):12593-8. doi: 10.1073/pnas.0909087107. Epub 2010 Jun 22. Proc Natl Acad Sci U S A. 2010. PMID: 20571119 Free PMC article.
-
N-terminus of hMLH1 confers interaction of hMutLalpha and hMutLbeta with hMutSalpha.Nucleic Acids Res. 2003 Jun 15;31(12):3217-26. doi: 10.1093/nar/gkg420. Nucleic Acids Res. 2003. PMID: 12799449 Free PMC article.
-
Identification of hMutLbeta, a heterodimer of hMLH1 and hPMS1.J Biol Chem. 1999 Nov 5;274(45):32368-75. doi: 10.1074/jbc.274.45.32368. J Biol Chem. 1999. PMID: 10542278
-
Molecular basis of HNPCC: mutations of MMR genes.Hum Mutat. 1997;10(2):89-99. doi: 10.1002/(SICI)1098-1004(1997)10:2<89::AID-HUMU1>3.0.CO;2-H. Hum Mutat. 1997. PMID: 9259192 Review.
-
Mismatch repair pathway: molecules, functions, and role in colorectal carcinogenesis.Eur J Cancer Prev. 2014 Jul;23(4):246-57. doi: 10.1097/CEJ.0000000000000019. Eur J Cancer Prev. 2014. PMID: 24614649 Review.
Cited by
-
Successful Correction by Prime Editing of a Mutation in the RYR1 Gene Responsible for a Myopathy.Cells. 2023 Dec 22;13(1):31. doi: 10.3390/cells13010031. Cells. 2023. PMID: 38201236 Free PMC article.
-
Expanded CAG/CTG repeats resist gene silencing mediated by targeted epigenome editing.Hum Mol Genet. 2022 Feb 3;31(3):386-398. doi: 10.1093/hmg/ddab255. Hum Mol Genet. 2022. PMID: 34494094 Free PMC article.
-
Antagonistic roles of canonical and Alternative-RPA in disease-associated tandem CAG repeat instability.Cell. 2023 Oct 26;186(22):4898-4919.e25. doi: 10.1016/j.cell.2023.09.008. Epub 2023 Oct 11. Cell. 2023. PMID: 37827155 Free PMC article.
-
Processing of double-R-loops in (CAG)·(CTG) and C9orf72 (GGGGCC)·(GGCCCC) repeats causes instability.Nucleic Acids Res. 2014;42(16):10473-87. doi: 10.1093/nar/gku658. Epub 2014 Aug 21. Nucleic Acids Res. 2014. PMID: 25147206 Free PMC article.
-
Replication stalling and DNA microsatellite instability.Biophys Chem. 2017 Jun;225:38-48. doi: 10.1016/j.bpc.2016.11.007. Epub 2016 Nov 22. Biophys Chem. 2017. PMID: 27914716 Free PMC article. Review.
References
-
- López Castel A., Cleary J. D., Pearson C. E. (2010) Repeat instability as the basis for human diseases and as a potential target for therapy. Nat. Rev. Mol. Cell Biol. 11, 165–170 - PubMed
-
- Slean M. M., Panigrahi G. B., Ranum L. P., Pearson C. E. (2008) Mutagenic roles of DNA “repair” proteins in antibody diversity and disease-associated trinucleotide repeat instability. DNA Repair 7, 1135–1154 - PubMed
-
- Manley K., Shirley T. L., Flaherty L., Messer A. (1999) Msh2 deficiency prevents in vivo somatic instability of the CAG repeat in Huntington disease transgenic mice. Nat. Genet. 23, 471–473 - PubMed
-
- Wheeler V. C., Lebel L. A., Vrbanac V., Teed A., te Riele H., MacDonald M. E. (2003) Mismatch repair gene Msh2 modifies the timing of early disease in Hdhq111 striatum. Hum. Mol. Genet. 12, 273–281 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

