Intracellular endothelin type B receptor-driven Ca2+ signal elicits nitric oxide production in endothelial cells
- PMID: 23086942
- PMCID: PMC3510805
- DOI: 10.1074/jbc.M112.418533
Intracellular endothelin type B receptor-driven Ca2+ signal elicits nitric oxide production in endothelial cells
Abstract
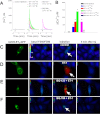
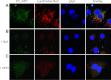
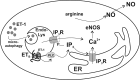
Endothelin-1 exerts its actions via activation of ET(A) and ET(B) G(q/11) protein-coupled receptors, located in the plasmalemma, cytoplasm, and nucleus. Although the autocrine/paracrine nature of endothelin-1 signaling has been extensively studied, its intracrine role has been largely attributed to interaction with receptors located on nuclear membranes and the nucleoplasm. Because ET(B) receptors have been shown to be targeted to endolysosomes, we used intracellular microinjection and concurrent imaging methods to test their involvement in Ca(2+) signaling and subsequential NO production. We provide evidence that microinjected endothelin-1 produces a dose-dependent elevation in cytosolic calcium concentration in ET(B)-transfected cells and endothelial cells; this response is sensitive to ET(B) but not ET(A) receptor blockade. In endothelial cells, the endothelin-1-induced Ca(2+) response is abolished upon endolysosomal but not Golgi disruption. Moreover, the effect is prevented by inhibition of microautophagy and is sensitive to inhibitors of the phospholipase C and inositol 1,4,5-trisphosphate receptor. Furthermore, intracellular endothelin-1 increases nitric oxide via an ET(B)-dependent mechanism. Our results indicate for the first time that intracellular endothelin-1 activates endolysosomal ET(B) receptors and increase cytosolic Ca(2+) and nitric oxide production. Endothelin-1 acts in an intracrine fashion on endolysosomal ET(B) to induce nitric oxide formation, thus modulating endothelial function.
Figures





Similar articles
-
Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome.Am J Pathol. 2014 Jun;184(6):1706-14. doi: 10.1016/j.ajpath.2014.02.027. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24731444 Free PMC article.
-
Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension.Circulation. 2012 Aug 21;126(8):963-74. doi: 10.1161/CIRCULATIONAHA.112.094722. Epub 2012 Jul 11. Circulation. 2012. PMID: 22787113 Free PMC article.
-
ET-1 induced Elevation of intracellular calcium in clonal neuronal and embryonic kidney cells involves endogenous endothelin-A receptors linked to phospholipase C through Gα(q/11).Pharmacol Res. 2011 Sep;64(3):258-67. doi: 10.1016/j.phrs.2011.04.003. Epub 2011 Apr 14. Pharmacol Res. 2011. PMID: 21515378 Free PMC article.
-
Impaired endothelin-induced vasoconstriction in coronary arteries of Zucker obese rats is associated with uncoupling of [Ca2+]i signaling.Am J Physiol Regul Integr Comp Physiol. 2006 Jan;290(1):R145-53. doi: 10.1152/ajpregu.00405.2005. Epub 2005 Dec 1. Am J Physiol Regul Integr Comp Physiol. 2006. PMID: 16322351
-
Role of endothelin-1 receptors in the sarcolemma membrane and the nuclear membrane in the modulation of basal cytosolic and nuclear calcium levels in heart cells.Clin Sci (Lond). 2002 Aug;103 Suppl 48:141S-147S. doi: 10.1042/CS103S141S. Clin Sci (Lond). 2002. PMID: 12193073
Cited by
-
Minimal contribution of IP3R2 in cardiac differentiation and derived ventricular-like myocytes from human embryonic stem cells.Acta Pharmacol Sin. 2020 Dec;41(12):1576-1586. doi: 10.1038/s41401-020-00528-w. Epub 2020 Oct 9. Acta Pharmacol Sin. 2020. PMID: 33037404 Free PMC article.
-
Hyaluronan Production by Renomedullary Interstitial Cells: Influence of Endothelin, Angiotensin II and Vasopressin.Int J Mol Sci. 2017 Dec 13;18(12):2701. doi: 10.3390/ijms18122701. Int J Mol Sci. 2017. PMID: 29236055 Free PMC article.
-
Endothelin 3/EDNRB signaling induces thermogenic differentiation of white adipose tissue.Nat Commun. 2024 Aug 22;15(1):7215. doi: 10.1038/s41467-024-51579-0. Nat Commun. 2024. PMID: 39174539 Free PMC article.
-
Differential activation of cultured neonatal cardiomyocytes by plasmalemmal versus intracellular G protein-coupled receptor 55.J Biol Chem. 2013 Aug 2;288(31):22481-92. doi: 10.1074/jbc.M113.456178. Epub 2013 Jun 27. J Biol Chem. 2013. PMID: 23814062 Free PMC article.
-
Endothelin-1 activation of the endothelin B receptor modulates pulmonary endothelial CX3CL1 and contributes to pulmonary angiogenesis in experimental hepatopulmonary syndrome.Am J Pathol. 2014 Jun;184(6):1706-14. doi: 10.1016/j.ajpath.2014.02.027. Epub 2014 Apr 13. Am J Pathol. 2014. PMID: 24731444 Free PMC article.
References
-
- Calebiro D., Nikolaev V. O., Lohse M. J. (2010) Imaging of persistent cAMP signaling by internalized G protein-coupled receptors. J. Mol. Endocrinol. 45, 1–8 - PubMed
-
- Re R. N., Cook J. L. (2007) Mechanisms of disease: Intracrine physiology in the cardiovascular system. Nat. Clin. Pract. Cardiovasc. Med. 4, 549–557 - PubMed
-
- Avedanian L., Riopel J., Bkaily G., Nader M., D'Orleans-Juste P., Jacques D. (2010) ETA receptors are present in human aortic vascular endothelial cells and modulate intracellular calcium. Can. J. Physiol. Pharmacol. 88, 817–829 - PubMed
-
- Bkaily G., Avedanian L., Al-Khoury J., Provost C., Nader M., D'Orléans-Juste P., Jacques D. (2011) Nuclear membrane receptors for ET-1 in cardiovascular function. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R251–263 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

