Melatonin inhibits nitric oxide signaling by increasing PDE5 phosphorylation in coronary arteries
- PMID: 23086989
- PMCID: PMC3532536
- DOI: 10.1152/ajpheart.00211.2012
Melatonin inhibits nitric oxide signaling by increasing PDE5 phosphorylation in coronary arteries
Abstract
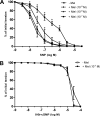
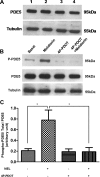
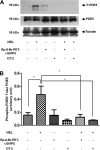
Melatonin inhibits nitric oxide (NO)-induced relaxation of coronary arteries. We tested the hypothesis that melatonin increases the phosphorylation of phosphodiesterase 5 (PDE5), which increases the activity of the enzyme and thereby decreases intracellular cGMP accumulation in response to NO and inhibits NO-induced relaxation. Sodium nitroprusside (SNP) and 8-Br-cGMP caused concentration-dependent relaxation of isolated coronary arteries suspended in organ chambers for isometric tension recording. In the presence of melatonin, the concentration-response curve to SNP, but not 8-Br-cGMP, was shifted to the right. The effect of melatonin on SNP-induced relaxation was abolished in the presence of the PDE5 inhibitors zaprinast and sildenafil. Melatonin markedly inhibited the SNP-induced increase in intracellular cGMP in coronary arteries, an effect that was also abolished by zaprinast. Treatment of coronary arteries with melatonin caused a nearly fourfold increase in the phosphorylation of PDE5, which increased the catalytic activity of the enzyme and thereby increased the degradation of cGMP to inactive 5'-GMP. Melatonin-induced PDE5 phosphorylation was markedly attenuated in the presence of the PKG1 inhibitors DT-2 or Rp-8-Br-PET-cGMPS and in those arteries in which PKG1 expression was first downregulated by 24-h incubation with SNP before exposure to melatonin. The selective MT(2) receptor antagonist 4-phenyl-2-propionamidotetralin completely blocked the stimulatory effect of melatonin on PDE5 phosphorylation as well as the inhibitory effect of melatonin on SNP-induced relaxation and intracellular cGMP. Thus, in coronary arteries, melatonin acts via MT(2) receptors and PKG1 to increase PDE5 phosphorylation, resulting in decreased cGMP accumulation in response to NO and impaired NO-induced vasorelaxation.
Figures




References
-
- Artemyev NO. Binding of transducin to light-activated rhodopsin prevents transducin interaction with the rod cGMP phosphodiesterase gamma-subunit. Biochemistry 36: 4188–4193, 1997 - PubMed
-
- Ballard SA, Gingell CJ, Tang K, Turner LA, Price ME, Naylor AM. Effects of sildenafil on the relaxation of human corpus cavernosum tissue in vitro and on the activities of cyclic nucleotide phosphodiesterase isozymes. J Urol 159: 2164–2171, 1998 - PubMed
-
- Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol Rev 58: 488–520, 2006 - PubMed
-
- Boolell M, Allen MJ, Ballard SA, Gepi-Attee S, Muirhead GJ, Naylor AM, Osterloh IH, Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res 8: 47–52, 1996 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

