Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice
- PMID: 23087000
- PMCID: PMC3595315
- DOI: 10.1126/science.1226929
Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice
Abstract
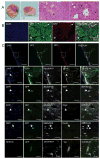
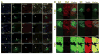
Glioblastoma multiforme (GBM) is the most common and aggressive malignant primary brain tumor in humans. Here we show that gliomas can originate from differentiated cells in the central nervous system (CNS), including cortical neurons. Transduction by oncogenic lentiviral vectors of neural stem cells (NSCs), astrocytes, or even mature neurons in the brains of mice can give rise to malignant gliomas. All the tumors, irrespective of the site of lentiviral vector injection (the initiating population), shared common features of high expression of stem or progenitor markers and low expression of differentiation markers. Microarray analysis revealed that tumors of astrocytic and neuronal origin match the mesenchymal GBM subtype. We propose that most differentiated cells in the CNS upon defined genetic alterations undergo dedifferentiation to generate a NSC or progenitor state to initiate and maintain the tumor progression, as well as to give rise to the heterogeneous populations observed in malignant gliomas.
Figures


Comment in
-
Glioma: Tumour cells in reverse.Nat Rev Cancer. 2012 Dec;12(12):794. doi: 10.1038/nrc3404. Nat Rev Cancer. 2012. PMID: 23175112 No abstract available.
-
Cancer. Can one cell influence cancer heterogeneity?Science. 2012 Nov 23;338(6110):1035-6. doi: 10.1126/science.1231594. Science. 2012. PMID: 23180850 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

