Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes
- PMID: 23087012
- PMCID: PMC3488745
- DOI: 10.1136/bmjopen-2012-001007
Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes
Abstract
Background: Despite the number of medications for type 2 diabetes, many people with the condition do not achieve good glycaemic control. Some existing glucose-lowering agents have adverse effects such as weight gain or hypoglycaemia. Type 2 diabetes tends to be a progressive disease, and most patients require treatment with combinations of glucose-lowering agents. The sodium glucose co-transporter 2 (SGLT2) receptor inhibitors are a new class of glucose-lowering agents.
Objective: To assess the clinical effectiveness and safety of the SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes.
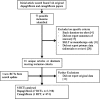
Data sources: MEDLINE, Embase, Cochrane Library (all sections); Science Citation Index; trial registries; conference abstracts; drug regulatory authorities; bibliographies of retrieved papers.
Inclusion criteria: Randomised controlled trials of SGLT2 receptor inhibitors compared with placebo or active comparator in type 2 diabetes in dual or combination therapy.
Methods: Systematic review. Quality assessment used the Cochrane risk of bias score.
Results: Seven trials, published in full, assessed dapagliflozin and one assessed canagliflozin. Trial quality appeared good. Dapagliflozin 10 mg reduced HbA1c by -0.54% (weighted mean differences (WMD), 95% CI -0.67 to -0.40) compared to placebo, but there was no difference compared to glipizide. Canagliflozin reduced HbA1c slightly more than sitagliptin (up to -0.21% vs sitagliptin). Both dapagliflozin and canagliflozin led to weight loss (dapagliflozin WMD -1.81 kg (95% CI -2.04 to -1.57), canagliflozin up to -2.3 kg compared to placebo).
Limitations: Long-term trial extensions suggested that effects were maintained over time. Data on canagliflozin are currently available from only one paper. Costs of the drugs are not known so cost-effectiveness cannot be assessed. More data on safety are needed, with the Food and Drug Administration having concerns about breast and bladder cancers.
Conclusions: Dapagliflozin appears effective in reducing HbA1c and weight in type 2 diabetes, although more safety data are needed.
Figures
References
-
- Diabetes UK. Diabetes in the UK: key statistics on diabetes. http://www.diabetes.org.uk/Documents/Reports/Diabetes_in_the_UK_2010.pdf 2010. (accessed 2 Aug 2012).
-
- Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76–9 - PubMed
-
- Stone PH, Muller JE, Hartwell T, et al. The effect of diabetes mellitus on prognosis and serial left ventricular function after acute myocardial infarction: contribution of both coronary disease and diastolic left ventricular dysfunction to the adverse prognosis. The MILIS Study Group. J Am Coll Cardiol 1989;14:49–57 - PubMed
-
- Santer R, Kinner M, Lassen CL, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 2003;14:2873–82 - PubMed
-
- Hanefeld M, Forst T. Dapagliflozin, an SGLT2 inhibitor, for diabetes. Lancet 2010;375:2196–8 - PubMed
LinkOut - more resources
Full Text Sources