High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression
- PMID: 23087017
- PMCID: PMC3543641
- DOI: 10.1152/ajplung.00342.2012
High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression
Retraction in
-
Retraction for Scott et al., volume 304, 2013, p. L70-L81.Am J Physiol Lung Cell Mol Physiol. 2021 Nov 1;321(5):L989. doi: 10.1152/ajplung.00342.2012_RET. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34748449 Free PMC article. No abstract available.
Abstract
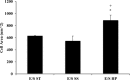
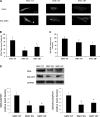
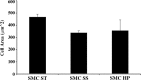
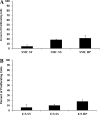
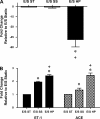
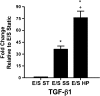
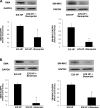
Proximal arterial stiffening is an important predictor of events in systemic and pulmonary hypertension, partly through its contribution to downstream vascular abnormalities. However, much remains undetermined regarding the mechanisms involved in the vascular changes induced by arterial stiffening. We therefore addressed the hypothesis that high pulsatility flow, caused by proximal arterial stiffening, induces downstream pulmonary artery endothelial cell (EC) dysfunction that in turn leads to phenotypic change of smooth muscle cells (SMCs). To test the hypothesis, we employed a model pulmonary circulation in which upstream compliance regulates the pulsatility of flow waves imposed onto a downstream vascular mimetic coculture composed of pulmonary ECs and SMCs. The effects of high pulsatility flow on SMCs were determined both in the presence and absence of ECs. In the presence of ECs, high pulsatility flow increased SMC size and expression of the contractile proteins, smooth muscle α-actin (SMA) and smooth muscle myosin heavy chain (SM-MHC), without affecting proliferation. In the absence of ECs, high pulsatility flow decreased SMC expression of SMA and SM-MHC, without affecting SMC size or proliferation. To identify the molecular signals involved in the EC-mediated SMC responses, mRNA and/or protein expression of vasoconstrictors [angiotensin-converting enzyme (ACE) and endothelin (ET)-1], vasodilator (eNOS), and growth factor (TGF-β1) in EC were examined. Results showed high pulsatility flow decreased eNOS and increased ACE, ET-1, and TGF-β1 expression. ACE inhibition with ramiprilat, ET-1 receptor inhibition with bosentan, and treatment with the vasodilator bradykinin prevented flow-induced, EC-dependent SMC changes. In conclusion, high pulsatility flow stimulated SMC hypertrophy and contractile protein expression by altering EC production of vasoactive mediators and cytokines, supporting the idea of a coupling between proximal vascular stiffening, flow pulsatility, and downstream vascular function.
Figures




References
-
- Adji A, O'Rourke MF, Namasivayam M. Arterial stiffness, its assessment, prognostic value, and implications for treatment. Am J Hypertens 24: 5–17, 2011 - PubMed
-
- Amann K, Gharehbaghi H, Stephen S, Mall G. Hypertrophy and hyperplasia of smooth muscle cells of small intramyocardial arteries in spontaneously hypertensive rats. Hypertension 25: 124–131, 1995 - PubMed
-
- Bakker EN, Matlung HL, Bonta P, de Vries CJ, van Rooijen N, Vanbavel E. Blood flow-dependent arterial remodelling is facilitated by inflammation but directed by vascular tone. Cardiovasc Res 78: 341–348, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

