Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2
- PMID: 23087021
- PMCID: PMC3526159
- DOI: 10.1093/hmg/dds427
Changes in Purkinje cell firing and gene expression precede behavioral pathology in a mouse model of SCA2
Abstract
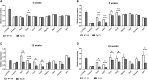
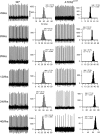
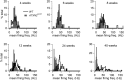
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominantly inherited disorder, which is caused by a pathological expansion of a polyglutamine (polyQ) tract in the coding region of the ATXN2 gene. Like other ataxias, SCA2 most overtly affects Purkinje cells (PCs) in the cerebellum. Using a transgenic mouse model expressing a full-length ATXN2(Q127)-complementary DNA under control of the Pcp2 promoter (a PC-specific promoter), we examined the time course of behavioral, morphologic, biochemical and physiological changes with particular attention to PC firing in the cerebellar slice. Although motor performance began to deteriorate at 8 weeks of age, reductions in PC number were not seen until after 12 weeks. Decreases in the PC firing frequency first showed at 6 weeks and paralleled deterioration of motor performance with progression of disease. Transcription changes in several PC-specific genes such as Calb1 and Pcp2 mirrored the time course of changes in PC physiology with calbindin-28 K changes showing the first small, but significant decreases at 4 weeks. These results emphasize that in this model of SCA2, physiological and behavioral phenotypes precede morphological changes by several weeks and provide a rationale for future studies examining the effects of restoration of firing frequency on motor function and prevention of future loss of PCs.
Figures





References
-
- Pulst S.M., Nechiporuk A., Nechiporuk T., Gispert S., Chen X. N., Lopes-Cendes I., Pearlman S., Starkman S., Orozco-Diaz G., Lunkes A., et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat. Genet. 1996;14:269–276. doi:10.1038/ng1196-269. - DOI - PubMed
-
- Pulst S.M., Santos N., Wang D., Yang H., Huynh D., Velazquez L., Figueroa K.P. Spinocerebellar ataxia type 2: polyQ repeat variation in the CACNA1A calcium channel modifies age of onset. Brain. 2005;128:2297–2303. doi:10.1093/brain/awh586. - DOI - PubMed
-
- van de Warrenburg B.P., Hendriks H., Durr A., van Zuijlen M.C., Stevanin G., Camuzat A., Sinke R.J., Brice A., Kremer B.P. Age at onset variance analysis in spinocerebellar ataxias: a study in a Dutch–French cohort. Ann. Neurol. 2005;57:505–512. doi:10.1002/ana.20424. - DOI - PubMed
-
- Servadio A., Koshy B., Armstrong D., Antalffy B., Orr H.T., Zoghbi H.Y. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat. Genet. 1995;10:94–98. doi:10.1038/ng0595-94. - DOI - PubMed
-
- Banfi S., Servadio A., Chung M., Capozzoli F., Duvick L.A., Elde R., Zoghbi H.Y., Orr H.T. Cloning and developmental expression analysis of the murine homolog of the spinocerebellar ataxia type 1 gene (Sca1) Hum. Mol. Genet. 1996;5:33–40. doi:10.1093/hmg/5.1.33. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

