Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection
- PMID: 23087212
- PMCID: PMC3521679
- DOI: 10.1091/mbc.E12-08-0619
Sequestration of G3BP coupled with efficient translation inhibits stress granules in Semliki Forest virus infection
Abstract
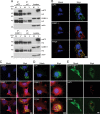
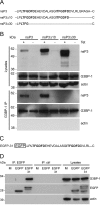
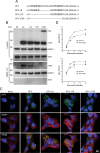
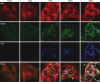
Dynamic, mRNA-containing stress granules (SGs) form in the cytoplasm of cells under environmental stresses, including viral infection. Many viruses appear to employ mechanisms to disrupt the formation of SGs on their mRNAs, suggesting that they represent a cellular defense against infection. Here, we report that early in Semliki Forest virus infection, the C-terminal domain of the viral nonstructural protein 3 (nsP3) forms a complex with Ras-GAP SH3-domain-binding protein (G3BP) and sequesters it into viral RNA replication complexes in a manner that inhibits the formation of SGs on viral mRNAs. A viral mutant carrying a C-terminal truncation of nsP3 induces more persistent SGs and is attenuated for propagation in cell culture. Of importance, we also show that the efficient translation of viral mRNAs containing a translation enhancer sequence also contributes to the disassembly of SGs in infected cells. Furthermore, we show that the nsP3/G3BP interaction also blocks SGs induced by other stresses than virus infection. This is one of few described viral mechanisms for SG disruption and underlines the role of SGs in antiviral defense.
Figures
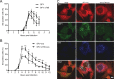
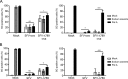
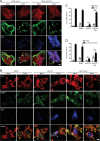
References
-
- Anderson P, Kedersha N. Stress granules. Curr Biol. 2009;19:R397–R398. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

