G9a mediates Sharp-1-dependent inhibition of skeletal muscle differentiation
- PMID: 23087213
- PMCID: PMC3521685
- DOI: 10.1091/mbc.E12-04-0311
G9a mediates Sharp-1-dependent inhibition of skeletal muscle differentiation
Abstract
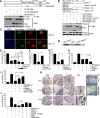
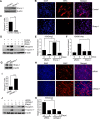
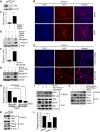
Sharp-1, a basic helix-loop-helix transcription factor, is a potent repressor of skeletal muscle differentiation and is dysregulated in muscle pathologies. However, the mechanisms by which it inhibits myogenesis are not fully understood. Here we show that G9a, a lysine methyltransferase, is involved in Sharp-1-mediated inhibition of muscle differentiation. We demonstrate that G9a directly interacts with Sharp-1 and enhances its ability to transcriptionally repress the myogenin promoter. Concomitant with a differentiation block, G9a-dependent histone H3 lysine 9 dimethylation (H3K9me2) and MyoD methylation are apparent upon Sharp-1 overexpression in muscle cells. RNA interference-mediated reduction of G9a or pharmacological inhibition of its activity erases these repressive marks and rescues the differentiation defect imposed by Sharp-1. Our findings provide new insights into Sharp-1-dependent regulation of myogenesis and identify epigenetic mechanisms that could be targeted in myopathies characterized by elevated Sharp-1 levels.
Figures

Similar articles
-
G9a promotes proliferation and inhibits cell cycle exit during myogenic differentiation.Nucleic Acids Res. 2016 Sep 30;44(17):8129-43. doi: 10.1093/nar/gkw483. Epub 2016 May 26. Nucleic Acids Res. 2016. PMID: 27229136 Free PMC article.
-
Sumoylation of the basic helix-loop-helix transcription factor sharp-1 regulates recruitment of the histone methyltransferase G9a and function in myogenesis.J Biol Chem. 2013 Jun 14;288(24):17654-62. doi: 10.1074/jbc.M113.463257. Epub 2013 Apr 30. J Biol Chem. 2013. PMID: 23637228 Free PMC article.
-
Lysine methyltransferase G9a methylates the transcription factor MyoD and regulates skeletal muscle differentiation.Proc Natl Acad Sci U S A. 2012 Jan 17;109(3):841-6. doi: 10.1073/pnas.1111628109. Epub 2012 Jan 3. Proc Natl Acad Sci U S A. 2012. PMID: 22215600 Free PMC article.
-
H3K9 methyltransferase G9a and the related molecule GLP.Genes Dev. 2011 Apr 15;25(8):781-8. doi: 10.1101/gad.2027411. Genes Dev. 2011. PMID: 21498567 Free PMC article. Review.
-
G9a, a multipotent regulator of gene expression.Epigenetics. 2013 Jan;8(1):16-22. doi: 10.4161/epi.23331. Epub 2012 Dec 20. Epigenetics. 2013. PMID: 23257913 Free PMC article. Review.
Cited by
-
Open access chemical probes for epigenetic targets.Future Med Chem. 2015;7(14):1901-17. doi: 10.4155/fmc.15.127. Epub 2015 Sep 23. Future Med Chem. 2015. PMID: 26397018 Free PMC article. Review.
-
G9a promotes proliferation and inhibits cell cycle exit during myogenic differentiation.Nucleic Acids Res. 2016 Sep 30;44(17):8129-43. doi: 10.1093/nar/gkw483. Epub 2016 May 26. Nucleic Acids Res. 2016. PMID: 27229136 Free PMC article.
-
Hypoxia-Inducible Lysine Methyltransferases: G9a and GLP Hypoxic Regulation, Non-histone Substrate Modification, and Pathological Relevance.Front Genet. 2020 Sep 3;11:579636. doi: 10.3389/fgene.2020.579636. eCollection 2020. Front Genet. 2020. PMID: 33088284 Free PMC article. Review.
-
Evolutionary history and epigenetic regulation of the three paralogous pax7 genes in rainbow trout.Cell Tissue Res. 2015 Mar;359(3):715-27. doi: 10.1007/s00441-014-2060-0. Epub 2014 Dec 10. Cell Tissue Res. 2015. PMID: 25487404 Free PMC article.
-
Parp3 assists muscle function and skeletal muscle differentiation by selectively adjusting H3K27me3 enrichment.iScience. 2025 Mar 25;28(4):112267. doi: 10.1016/j.isci.2025.112267. eCollection 2025 Apr 18. iScience. 2025. PMID: 40248123 Free PMC article.
References
-
- Azmi S, Ozog A, Taneja R. Sharp-1/DEC2 inhibits skeletal muscle differentiation through repression of myogenic transcription factors. J Biol Chem. 2004;279:52643–52652. - PubMed
-
- Azmi S, Sun H, Ozog A, Taneja R. mSharp-1/DEC2, a basic helix-loop-helix protein functions as a transcriptional repressor of E box activity and Stra13 expression. J Biol Chem. 2003;278:20098–20109. - PubMed
-
- Azmi S, Taneja R. Embryonic expression of mSharp-1/mDEC2, which encodes a basic helix-loop-helix transcription factor. Mech Dev. 2002;114:181–185. - PubMed
-
- Chen L, Zhou J, Xu H, Xu G, Xue J. Identification and developmental expression of Dec2 in zebrafish. Fish Physiol Biochem. 2010;36:667–675. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

