Blood clots are rapidly assembled hemodynamic sensors: flow arrest triggers intraluminal thrombus contraction
- PMID: 23087356
- PMCID: PMC3586804
- DOI: 10.1161/ATVBAHA.112.300312
Blood clots are rapidly assembled hemodynamic sensors: flow arrest triggers intraluminal thrombus contraction
Abstract
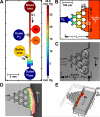
Objective: Blood clots form under flow during intravascular thrombosis or vessel leakage. Prevailing hemodynamics influence thrombus structure and may regulate contraction processes. A microfluidic device capable of flowing human blood over a side channel plugged with collagen (± tissue factor) was used to measure thrombus permeability (κ) and contraction at controlled transthrombus pressure drops.
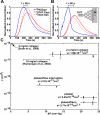
Methods and results: The collagen (κ(collagen)=1.98 × 10(-11) cm(2)) supported formation of a 20-µm thick platelet layer, which unexpectedly underwent massive platelet retraction on flow arrest. This contraction resulted in a 5.34-fold increase in permeability because of collagen restructuring. Without stopping flow, platelet deposits (no fibrin) had a permeability of κ(platelet)=5.45 × 10(-14) cm(2) and platelet-fibrin thrombi had κ(thrombus)=2.71 × 10(-14) cm(2) for ΔP=20.7 to 23.4 mm Hg, the first ever measurements for clots formed under arterial flow (1130 s(-1) wall shear rate). Platelet sensing of flow cessation triggered a 4.6- to 6.5-fold (n=3, P<0.05) increase in contraction rate, which was also observed in a rigid, impermeable parallel-plate microfluidic device. This triggered contraction was blocked by the myosin IIA inhibitor blebbistatin and by inhibitors of thromboxane A2 (TXA(2)) and ADP signaling. In addition, flow arrest triggered platelet intracellular calcium mobilization, which was blocked by TXA(2)/ADP inhibitors. As clots become occlusive or blood pools following vessel leakage, the flow diminishes, consequently allowing full platelet retraction.
Conclusions: Flow dilution of ADP and thromboxane regulates platelet contractility with prevailing hemodynamics, a newly defined flow-sensing mechanism to regulate clot function.
Figures
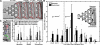

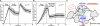
References
-
- Laurens N, Koolwij P, De Maat MPM. Fibrin structure and wound healing. J Thromb Heamost. 2006;4:932–939. - PubMed
-
- Jen CJ, McIntrie LV. The structural properties and contractile force of a clot. Cell Motil. 1982;2:445–455. - PubMed
-
- Adelstein RS. Calmodulin and the regulation of the actin-myosin interaction in smooth muscle and nonmuscle cells. Cell. 1982;30:349–350. - PubMed
-
- Suzuki Y, Yamamoto M, Wada H, Ito M, Nakano T, Sasaki Y, Narumiya S, Shiku H, Nishikawa M. Agonist-induced regulation of myosin phosphatase activity in human platelets through activation of Rho-kinase. Blood. 1999;93:3408–3417. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

