Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns Sayre Syndrome
- PMID: 23087375
- PMCID: PMC3592399
- DOI: 10.1093/nar/gks965
Mitochondrial targeting of recombinant RNAs modulates the level of a heteroplasmic mutation in human mitochondrial DNA associated with Kearns Sayre Syndrome
Abstract
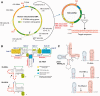
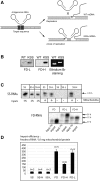
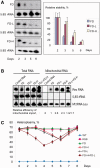
Mitochondrial mutations, an important cause of incurable human neuromuscular diseases, are mostly heteroplasmic: mutated mitochondrial DNA is present in cells simultaneously with wild-type genomes, the pathogenic threshold being generally >70% of mutant mtDNA. We studied whether heteroplasmy level could be decreased by specifically designed oligoribonucleotides, targeted into mitochondria by the pathway delivering RNA molecules in vivo. Using mitochondrially imported RNAs as vectors, we demonstrated that oligoribonucleotides complementary to mutant mtDNA region can specifically reduce the proportion of mtDNA bearing a large deletion associated with the Kearns Sayre Syndrome in cultured transmitochondrial cybrid cells. These findings may be relevant to developing of a new tool for therapy of mtDNA associated diseases.
Figures


References
-
- Wallace DC. Mitochondrial diseases in man and mouse. Science. 1999;283:1482–1488. - PubMed
-
- Kolesnikova OA, Entelis NS, Mireau H, Fox TD, Martin RP, Tarassov IA. Suppression of mutations in mitochondrial DNA by tRNAs imported from the cytoplasm. Science. 2000;289:1931–1933. - PubMed
-
- Mahata B, Mukherjee S, Mishra S, Bandyopadhyay A, Adhya S. Functional delivery of a cytosolic tRNA into mutant mitochondria of human cells. Science. 2006;314:471–474. - PubMed
-
- Manfredi G, Fu J, Ojaimi J, Sadlock JE, Kwong JQ, Guy J, Schon EA. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nat. Genet. 2002;30:394–399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

