1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans
- PMID: 23087428
- PMCID: PMC3523798
- DOI: 10.1093/infdis/jis652
1976 and 2009 H1N1 influenza virus vaccines boost anti-hemagglutinin stalk antibodies in humans
Abstract
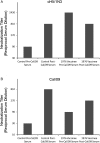
Background: Infection with pandemic H1N1 influenza A viruses (IAVs) containing hemagglutinin (HA) proteins with globular heads that differ substantially from seasonal strains results in a boost in broadly cross-reactive antibodies that bind to the HA stalk. Boosting these antibodies has become an attractive strategy for creating a universal IAV vaccine. Therefore, it was essential to determine whether vaccines containing H1N1 viruses whose head domains differ substantially compared to seasonal strains could also achieve this boost.
Methods: Prospective samples of subjects who had received the A/New Jersey/1976 (NJ/76) vaccine and healthy, age-matched controls were assessed for the presence of anti-HA stalk antibodies before and after receiving the A/California/04/2009 (Cal/09) vaccine between October 2009 and January 2010.
Results: Individuals who received either the NJ/76 vaccine or the Cal/09 vaccine experienced a robust boost in HA stalk-reactive, neutralizing antibodies similar to what has been observed in individuals infected with Cal/09.
Conclusions: These results demonstrate that vaccines containing viruses whose HA head domains that differ substantially from seasonal strains are capable of boosting titers of HA stalk antibodies. Furthermore, anti-HA stalk antibodies elicited by vaccination appear to be long-lived and therefore could be targeted for the generation of a universal IAV vaccine.
Figures


Similar articles
-
Impact of pre-existing immunity on the induction of functional cross-reactive anti-hemagglutinin stalk antibodies following vaccination with an AS03 adjuvanted pandemic H1N1 vaccine.Vaccine. 2018 Apr 12;36(16):2213-2219. doi: 10.1016/j.vaccine.2018.02.022. Epub 2018 Mar 13. Vaccine. 2018. PMID: 29548607
-
Induction of broadly reactive anti-hemagglutinin stalk antibodies by an H5N1 vaccine in humans.J Virol. 2014 Nov;88(22):13260-8. doi: 10.1128/JVI.02133-14. Epub 2014 Sep 10. J Virol. 2014. PMID: 25210189 Free PMC article.
-
Influenza Virus Hemagglutinin Stalk-Specific Antibodies in Human Serum are a Surrogate Marker for In Vivo Protection in a Serum Transfer Mouse Challenge Model.mBio. 2017 Sep 19;8(5):e01463-17. doi: 10.1128/mBio.01463-17. mBio. 2017. PMID: 28928215 Free PMC article.
-
Novel universal influenza virus vaccine approaches.Curr Opin Virol. 2016 Apr;17:95-103. doi: 10.1016/j.coviro.2016.02.002. Epub 2016 Feb 27. Curr Opin Virol. 2016. PMID: 26927813 Free PMC article. Review.
-
The Quest for a Truly Universal Influenza Vaccine.Front Cell Infect Microbiol. 2019 Oct 10;9:344. doi: 10.3389/fcimb.2019.00344. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31649895 Free PMC article. Review.
Cited by
-
Dynamics of influenza in tropical Africa: Temperature, humidity, and co-circulating (sub)types.Influenza Other Respir Viruses. 2018 Jul;12(4):446-456. doi: 10.1111/irv.12556. Epub 2018 Apr 17. Influenza Other Respir Viruses. 2018. PMID: 29573157 Free PMC article.
-
Options and obstacles for designing a universal influenza vaccine.Viruses. 2014 Aug 18;6(8):3159-80. doi: 10.3390/v6083159. Viruses. 2014. PMID: 25196381 Free PMC article. Review.
-
Infection with 2009 H1N1 influenza virus primes for immunological memory in human nose-associated lymphoid tissue, offering cross-reactive immunity to H1N1 and avian H5N1 viruses.J Virol. 2013 May;87(10):5331-9. doi: 10.1128/JVI.03547-12. Epub 2013 Mar 6. J Virol. 2013. PMID: 23468498 Free PMC article.
-
Antibody Response to Influenza Hemagglutinin Conserved Stalk Domain after Sequential Immunization with Old Vaccine Strains.Adv Virol. 2024 Feb 13;2024:5691673. doi: 10.1155/2024/5691673. eCollection 2024. Adv Virol. 2024. PMID: 38379638 Free PMC article.
-
Adjuvants and immunization strategies to induce influenza virus hemagglutinin stalk antibodies.PLoS One. 2013 Nov 6;8(11):e79194. doi: 10.1371/journal.pone.0079194. eCollection 2013. PLoS One. 2013. PMID: 24223176 Free PMC article.
References
-
- Palese P, Shaw ML. Orthomyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Field's Virology. 5th ed. Vol. 2. Philadelphia: Lippincott; 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

