Localized mucosal response to intranasal live attenuated influenza vaccine in adults
- PMID: 23087433
- PMCID: PMC3571238
- DOI: 10.1093/infdis/jis641
Localized mucosal response to intranasal live attenuated influenza vaccine in adults
Abstract
Background: Influenza virus infection is a major public health burden worldwide. Available vaccines include the inactivated intramuscular trivalent vaccine and, more recently, an intranasal live attenuated influenza vaccine (LAIV). The measure of successful vaccination with the inactivated vaccine is a systemic rise in immunoglobulin G (IgG) level, but for the LAIV no such correlate has been established.
Methods: Seventy-nine subjects were given the LAIV FluMist. Blood was collected prior to vaccination and 3 days and 30 days after vaccination. Nasal wash was collected 3 days and 30 days after vaccination. Responses were measured systemically and in mucosal secretions for cytokines, cell activation profiles, and antibody responses.
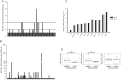
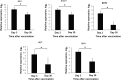
Results: Only 9% of subjects who received LAIV seroconverted, while 33% of patients developed at least a 2-fold increase in influenza virus-specific immunoglobulin A (IgA) antibodies in nasal wash. LAIV induced a localized inflammation, as suggested by increased expression of interferon-response genes in mucosal RNA and increased granulocyte colony-stimulating factor (G-CSF) and IP-10 in nasal wash. Interestingly, patients who seroconverted had significantly lower serum levels of G-CSF before vaccination.
Conclusions: Protection by LAIV is likely provided through mucosal IgA and not by increases in systemic IgG. LAIV induces local inflammation. Seroconversion is achieved in a small fraction of subjects with a lower serum G-CSF level.
Figures


References
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. - PubMed
-
- Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–62. - PubMed
-
- Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vaccines. 2004;3:643–54. - PubMed
-
- Esposito S, Montinaro V, Groppali E, Tenconi R, Semino M, Principi N. Live attenuated intranasal influenza vaccine. Hum Vaccin Immunother. 2012;8:1–5. - PubMed
-
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71:1591–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

