The in vitro anti-viral potential of Setarud (IMOD™), a commercial herbal medicine with protective activity against acquired immune deficiency syndrome in clinical trials
- PMID: 23087503
- PMCID: PMC3469945
- DOI: 10.4103/0253-7613.99301
The in vitro anti-viral potential of Setarud (IMOD™), a commercial herbal medicine with protective activity against acquired immune deficiency syndrome in clinical trials
Abstract
Objectives: Setarud (IMOD™) is a herbal medicine with beneficial effect for patients suffering Human immunodeficiency virus (HIV) infection and has been approved for IV (intra venues) injection. The beneficial effect of IMOD administration for acquired immune deficiency syndrome (AIDS) patient has been proved in previous clinical trials. Here the in vitro inhibitory effect of IMOD against HIV-1, Herpes simplex virus (HSV) and murine leukemia viruses (MLV) was evaluated.
Materials and methods: HIV single cycle replication and HSV plaque reduction assays were used to evaluate the anti-viral effect. The level of HIV replication was monitored by p24 capture Enzyme-linked immunosorbent assay (ELISA). The single round infection [with green fluorescent protein (GFP) reporter MLV and HIV], virucidal and time-of-additions (HSV) assays were utilized to determine the mode of anti-viral activity. The toxicity of IMOD for cells was monitored by XTT (sodium 3_-[1 (phenylaminocarbonyl)- 3,4-tetrazolium]-bis (4-methoxy-6-nitro)benzene sulfonic acid) cell proliferation assay kit.
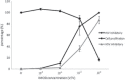
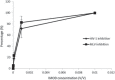
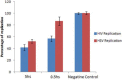
Results: IMOD inhibited 50% of HIV-1 and HSV replication (IC(50)) at 6.5 × 10(-4) and 4.3 × 10(-3)V/V concentrations, respectively. The IC(50) value against HIV-1 and MLV infection were 6 × 10(-4)V/V and 4.9 × 10(-4)V/V. Virucidal assay showed that IMOD reduces the potency of HIV and HSV particles to 41 and 54% of control, respectively. Time-of-addition study revealed that IMOD inhibits the replication of HSV at a stage after penetration of virions to the target cells.
Conclusions: Data from this study indicate that IMOD has significant anti-viral activity against HIV, HSV and MLV. Setarud could be subjected to further investigation after isolation of the constituents and determination of the toxic components.
Keywords: Anti-viral activity; human immunodeficiency virus; setarud (IMOD™).
Conflict of interest statement
Figures

Similar articles
-
Herbal Gel Formulation Developed for Anti-Human Immunodeficiency Virus (HIV)-1 Activity Also Inhibits In Vitro HSV-2 Infection.Viruses. 2018 Oct 24;10(11):580. doi: 10.3390/v10110580. Viruses. 2018. PMID: 30352961 Free PMC article.
-
Anti-HSV-2 activity of Terminalia chebula Retz extract and its constituents, chebulagic and chebulinic acids.BMC Complement Altern Med. 2017 Feb 14;17(1):110. doi: 10.1186/s12906-017-1620-8. BMC Complement Altern Med. 2017. PMID: 28196487 Free PMC article.
-
Venom Components of Iranian Scorpion Hemiscorpius lepturus Inhibit the Growth and Replication of Human Immunodeficiency Virus 1 (HIV-1).Iran Biomed J. 2016 Nov;20(5):259-65. doi: 10.22045/ibj.2016.02. Epub 2016 Sep 4. Iran Biomed J. 2016. PMID: 27594443 Free PMC article.
-
Safety and efficacy of Setarud (IMOD TM ) among people living with HIV/AIDS: a review.Recent Pat Antiinfect Drug Discov. 2012 Apr;7(1):66-72. doi: 10.2174/157489112799829756. Recent Pat Antiinfect Drug Discov. 2012. PMID: 22353002 Review.
-
Sulfated polysaccharides extracted from sea algae as potential antiviral drugs.Gen Pharmacol. 1997 Oct;29(4):497-511. doi: 10.1016/s0306-3623(96)00563-0. Gen Pharmacol. 1997. PMID: 9352294 Review.
Cited by
-
Toward the development of a single-round infection assay based on EGFP reporting for anti-HIV-1 drug discovery.Rep Biochem Mol Biol. 2015 Oct;4(1):1-9. Rep Biochem Mol Biol. 2015. PMID: 26989744 Free PMC article.
-
The effects of setarud on the immunological status of HIV-positive patients: Efficacy of a novel multi-herbal drug.Avicenna J Phytomed. 2017 May-Jun;7(3):232-241. Avicenna J Phytomed. 2017. PMID: 28748170 Free PMC article.
-
COVID-19 Prophylaxis Efforts Based on Natural Antiviral Plant Extracts and Their Compounds.Molecules. 2021 Jan 30;26(3):727. doi: 10.3390/molecules26030727. Molecules. 2021. PMID: 33573318 Free PMC article. Review.
-
Protective Effect of Selenium-Based Medicines on Toxicity of Three Common Organophosphorus Compounds in Human Erythrocytes In Vitro.Cell J. 2016 Winter;17(4):740-747. doi: 10.22074/cellj.2016.3846. Epub 2016 Jan 17. Cell J. 2016. PMID: 26862533 Free PMC article.
References
-
- Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–71. - PubMed
-
- Arora DR, Gautam V, Gill PS, Mishra N. Recent advances in antiretroviral therapy in HIV infection. J Indian Med Assoc. 2010;108:29–34. - PubMed
-
- Wilkin TJ, Shalev N, Tieu HV, Hammer SM. Advances in antiretroviral therapy. Top HIV Med. 2010;18:66–92. - PubMed
-
- Cohen J. HIV/AIDS. Tangled patent dispute over ‘free’ drug-resistance database. Science. 2009;323:1156–7. - PubMed
-
- Obiako OR, Murktar HM, Ogoina D. Antiretroviral drug resistance--implications for HIV/AIDS reduction in sub-saharan Africa and other developing countries. Niger J Med. 2010;19:302–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

