Translocating the blood-brain barrier using electrostatics
- PMID: 23087614
- PMCID: PMC3468918
- DOI: 10.3389/fncel.2012.00044
Translocating the blood-brain barrier using electrostatics
Abstract
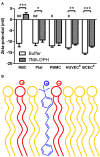
Mammalian cell membranes regulate homeostasis, protein activity, and cell signaling. The charge at the membrane surface has been correlated with these key events. Although mammalian cells are known to be slightly anionic, quantitative information on the membrane charge and the importance of electrostatic interactions in pharmacokinetics and pharmacodynamics remain elusive. Recently, we reported for the first time that brain endothelial cells (EC) are more negatively charged than human umbilical cord cells, using zeta-potential measurements by dynamic light scattering. Here, we hypothesize that anionicity is a key feature of the blood-brain barrier (BBB) and contributes to select which compounds cross into the brain. For the sake of comparison, we also studied the membrane surface charge of blood components-red blood cells (RBC), platelets, and peripheral blood mononuclear cells (PBMC). To further quantitatively correlate the negative zeta-potential values with membrane charge density, model membranes with different percentages of anionic lipids were also evaluated. From all the cells tested, brain cell membranes are the most anionic and those having their lipids mostly exposed, which explains why lipophilic cationic compounds are more prone to cross the blood-brain barrier.
Keywords: blood cells; blood-brain barrier; cell surface charge; drug targeting; zeta-potential.
Figures



Similar articles
-
Lidocaine turns the surface charge of biological membranes more positive and changes the permeability of blood-brain barrier culture models.Biochim Biophys Acta Biomembr. 2019 Sep 1;1861(9):1579-1591. doi: 10.1016/j.bbamem.2019.07.008. Epub 2019 Jul 10. Biochim Biophys Acta Biomembr. 2019. PMID: 31301276
-
Nanoparticle surface charges alter blood-brain barrier integrity and permeability.J Drug Target. 2004;12(9-10):635-41. doi: 10.1080/10611860400015936. J Drug Target. 2004. PMID: 15621689
-
Effect of cholesterol on the ion-membrane interaction: Zeta potential and dynamic light scattering study.Chem Phys Lipids. 2023 Aug;254:105307. doi: 10.1016/j.chemphyslip.2023.105307. Epub 2023 May 13. Chem Phys Lipids. 2023. PMID: 37182823
-
Surface charge, glycocalyx, and blood-brain barrier function.Tissue Barriers. 2021 Jul 3;9(3):1904773. doi: 10.1080/21688370.2021.1904773. Epub 2021 May 18. Tissue Barriers. 2021. PMID: 34003072 Free PMC article. Review.
-
Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review.Histol Histopathol. 2004 Apr;19(2):535-64. doi: 10.14670/HH-19.535. Histol Histopathol. 2004. PMID: 15024715 Review.
Cited by
-
Enhanced tumour penetration and prolonged circulation in blood of polyzwitterion-drug conjugates with cell-membrane affinity.Nat Biomed Eng. 2021 Sep;5(9):1019-1037. doi: 10.1038/s41551-021-00701-4. Epub 2021 Apr 15. Nat Biomed Eng. 2021. PMID: 33859387
-
Nanotechnology-based Drug Delivery for Alzheimer's and Parkinson's Diseases.Curr Drug Deliv. 2024;21(7):917-931. doi: 10.2174/1567201820666230707113405. Curr Drug Deliv. 2024. PMID: 37424345 Review.
-
Applied Electric Fields Polarize Initiation and Growth of Endothelial Sprouts.J Tissue Eng Regen Med. 2023 Dec 23;2023:6331148. doi: 10.1155/2023/6331148. eCollection 2023. J Tissue Eng Regen Med. 2023. PMID: 40226427 Free PMC article.
-
The Use of Sensors in Blood-Brain Barrier-on-a-Chip Devices: Current Practice and Future Directions.Biosensors (Basel). 2023 Mar 8;13(3):357. doi: 10.3390/bios13030357. Biosensors (Basel). 2023. PMID: 36979569 Free PMC article. Review.
-
Blood-Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience.Pharmaceuticals (Basel). 2024 May 10;17(5):612. doi: 10.3390/ph17050612. Pharmaceuticals (Basel). 2024. PMID: 38794182 Free PMC article. Review.
References
-
- Anderson J. (1981). Concentration dependence of electrophoretic mobility. J. Colloid Interface Sci. 82, 248–250
-
- Buser C. A., Sigal C. T., Resh M. D., McLaughlin S. (1994). Membrane binding of myristylated peptides corresponding to the NH2 terminus of Src. Biochemistry 33, 13093–13101 - PubMed
-
- Cansell M., Gouygou J. P., Jozefonvicz J., Letourneur D. (1997). Lipid composition of cultured endothelial cells in relation to their growth. Lipids 32, 39–44 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

