Modulation of physiological reflexes by pain: role of the locus coeruleus
- PMID: 23087627
- PMCID: PMC3474280
- DOI: 10.3389/fnint.2012.00094
Modulation of physiological reflexes by pain: role of the locus coeruleus
Abstract
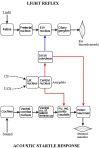
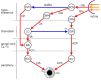
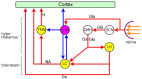
The locus coeruleus (LC) is activated by noxious stimuli, and this activation leads to inhibition of perceived pain. As two physiological reflexes, the acoustic startle reflex and the pupillary light reflex, are sensitive to noxious stimuli, this review considers evidence that this sensitivity, at least to some extent, is mediated by the LC. The acoustic startle reflex, contraction of a large body of skeletal muscles in response to a sudden loud acoustic stimulus, can be enhanced by both directly ("sensitization") and indirectly ("fear conditioning") applied noxious stimuli. Fear-conditioning involves the association of a noxious (unconditioned) stimulus with a neutral (conditioned) stimulus (e.g., light), leading to the ability of the conditioned stimulus to evoke the "pain response". The enhancement of the startle response by conditioned fear ("fear-potentiated startle") involves the activation of the amygdala. The LC may also be involved in both sensitization and fear potentiation: pain signals activate the LC both directly and indirectly via the amygdala, which results in enhanced motoneurone activity, leading to an enhanced muscular response. Pupil diameter is under dual sympathetic/parasympathetic control, the sympathetic (noradrenergic) output dilating, and the parasympathetic (cholinergic) output constricting the pupil. The light reflex (constriction of the pupil in response to a light stimulus) operates via the parasympathetic output. The LC exerts a dual influence on pupillary control: it contributes to the sympathetic outflow and attenuates the parasympathetic output by inhibiting the Edinger-Westphal nucleus, the preganglionic cholinergic nucleus in the light reflex pathway. Noxious stimulation results in pupil dilation ("reflex dilation"), without any change in the light reflex response, consistent with sympathetic activation via the LC. Conditioned fear, on the other hand, results in the attenuation of the light reflex response ("fear-inhibited light reflex"), consistent with the inhibition of the parasympathetic light reflex via the LC. It is suggested that directly applied pain and fear-conditioning may affect different populations of autonomic neurones in the LC, directly applied pain activating sympathetic and fear-conditioning parasympathetic premotor neurones.
Keywords: acoustic startle reflex; fear-conditioning; locus coeruleus; pain; pupillary light reflex.
Figures
Similar articles
-
Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways.Front Neurol. 2018 Dec 18;9:1069. doi: 10.3389/fneur.2018.01069. eCollection 2018. Front Neurol. 2018. PMID: 30619035 Free PMC article. Review.
-
Pupillary Reflexes in Complex Regional Pain Syndrome: Asymmetry to Arousal Stimuli Suggests an Ipsilateral Locus Coeruleus Deficit.J Pain. 2022 Jan;23(1):131-140. doi: 10.1016/j.jpain.2021.07.003. Epub 2021 Aug 8. J Pain. 2022. PMID: 34375745
-
Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions.Cell Rep. 2017 Sep 26;20(13):3099-3112. doi: 10.1016/j.celrep.2017.08.094. Cell Rep. 2017. PMID: 28954227 Free PMC article.
-
Sympathetic nervous system does not mediate reflex pupillary dilation during desflurane anesthesia.Anesthesiology. 1996 Oct;85(4):748-54. doi: 10.1097/00000542-199610000-00009. Anesthesiology. 1996. PMID: 8873544
-
Pharmacological analysis of fear-potentiated startle.Braz J Med Biol Res. 1993 Mar;26(3):235-60. Braz J Med Biol Res. 1993. PMID: 8257926 Review.
Cited by
-
Functional Organization of the Sympathetic Pathways Controlling the Pupil: Light-Inhibited and Light-Stimulated Pathways.Front Neurol. 2018 Dec 18;9:1069. doi: 10.3389/fneur.2018.01069. eCollection 2018. Front Neurol. 2018. PMID: 30619035 Free PMC article. Review.
-
Pupillometry: Psychology, Physiology, and Function.J Cogn. 2018 Feb 21;1(1):16. doi: 10.5334/joc.18. J Cogn. 2018. PMID: 31517190 Free PMC article.
-
Eye pupil - a window into central autonomic regulation via emotional/cognitive processing.Physiol Res. 2021 Dec 30;70(Suppl4):S669-S682. doi: 10.33549/physiolres.934749. Physiol Res. 2021. PMID: 35199551 Free PMC article. Review.
-
Psychological states affecting initial pupil size changes after olfactory stimulation in healthy participants.Sci Rep. 2023 Sep 25;13(1):16050. doi: 10.1038/s41598-023-43004-1. Sci Rep. 2023. PMID: 37749199 Free PMC article.
-
Suppression of pupillary unrest by general anesthesia and propofol sedation.J Clin Monit Comput. 2019 Apr;33(2):317-323. doi: 10.1007/s10877-018-0147-y. Epub 2018 May 21. J Clin Monit Comput. 2019. PMID: 29785552
References
-
- Adams L. M., Geyer M. A. (1981). Effects of 6-hydroxydopamine lesions of locus coeruleus on startle in rats. Psychopharmacol. (Berl.) 73, 394–398 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

