Obstructive sleep apnea and non-alcoholic Fatty liver disease: is the liver another target?
- PMID: 23087670
- PMCID: PMC3473309
- DOI: 10.3389/fneur.2012.00149
Obstructive sleep apnea and non-alcoholic Fatty liver disease: is the liver another target?
Abstract
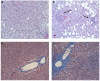
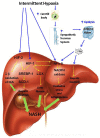
Obstructive sleep apnea (OSA) is recurrent obstruction of the upper airway during sleep leading to intermittent hypoxia (IH). OSA has been associated with all components of the metabolic syndrome as well as with non-alcoholic fatty liver disease (NAFLD). NAFLD is a common condition ranging in severity from uncomplicated hepatic steatosis to steatohepatitis (NASH), liver fibrosis, and cirrhosis. The gold standard for the diagnosis and staging of NAFLD is liver biopsy. Obesity and insulin resistance lead to liver steatosis, but the causes of the progression to NASH are not known. Emerging evidence suggests that OSA may play a role in the progression of hepatic steatosis and the development of NASH. Several cross-sectional studies showed that the severity of IH in patients with OSA predicted the severity of NAFLD on liver biopsy. However, neither prospective nor interventional studies with continuous positive airway pressure treatment have been performed. Studies in a mouse model showed that IH causes triglyceride accumulation in the liver and liver injury as well as hepatic inflammation. The mouse model provided insight in the pathogenesis of liver injury showing that (1) IH accelerates the progression of hepatic steatosis by inducing adipose tissue lipolysis and increasing free fatty acids (FFA) flux into the liver; (2) IH up-regulates lipid biosynthetic pathways in the liver; (3) IH induces oxidative stress in the liver; (4) IH up-regulates hypoxia inducible factor 1 alpha and possibly HIF-2 alpha, which may increase hepatic steatosis and induce liver inflammation and fibrosis. However, the role of FFA and different transcription factors in the pathogenesis of IH-induced NAFLD is yet to be established. Thus, multiple lines of evidence suggest that IH of OSA may contribute to the progression of NAFLD but definitive clinical studies and experiments in the mouse model have yet to be done.
Keywords: intermittent hypoxia; non-alcoholic fatty liver disease; non-alcoholic steatohepatitis; sleep apnea.
Figures
References
-
- Bao G., Metreveli N., Li R., Taylor A., Fletcher E. C. (1997). Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J. Appl. Physiol. 83, 95–101 - PubMed
LinkOut - more resources
Full Text Sources

