Recent advances in Drosophila male germline stem cell biology
- PMID: 23087833
- PMCID: PMC3469437
- DOI: 10.4161/spmg.21763
Recent advances in Drosophila male germline stem cell biology
Abstract
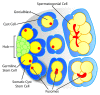
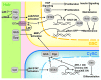
The ability of stem cells to divide asymmetrically to produce both self-renewing and differentiating daughter cells sustains many adult tissues, but germline stem cells (GSCs) are unique among stem cells as they perpetuate the genome of the species. The cellular and molecular mechanisms regulating most mammalian stem cells in their endogenous local microenvironments, or niches, are quite challenging to study. However, studies of stem cell niches such as those found in the Drosophila gonads have proven very useful. In these tissues, GSCs are housed in a readily identifiable niche, and the ability to genetically manipulate these cells and their neighbors has uncovered several fundamental mechanisms that are relevant to stem cells more generally. Here, we summarize recent work on the regulation of GSCs in the Drosophila testis niche by intercellular signals, and on the intracellular mechanisms that cooperate with these signals to ensure the survival of the germline. This review focuses on GSCs within the adult Drosophila testis; somatic stem cells in this tissue are reviewed by Zoller and Schulz in this issue.(1) For a review of the testis niche as a whole, see de Cuevas and Matunis,(2) and for more comprehensive reviews of the Drosophila testis, refer to Fuller(3) and Davies and Fuller.(4).
Figures
References
-
- Fuller MT. Spermatogenesis. In: Bate M, Martinez Arias A, eds. The Development of Drosphila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 1993:71-147.
-
- Whitworth C, Jimenez E, Van Doren M. Development of sexual dimorphism in the Drosophila testis. Spermatogenesis. 2012;3:1–8.
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
