Diverse phosphorylation patterns of B cell receptor-associated signaling in naïve and memory human B cells revealed by phosphoflow, a powerful technique to study signaling at the single cell level
- PMID: 23087912
- PMCID: PMC3473368
- DOI: 10.3389/fcimb.2012.00128
Diverse phosphorylation patterns of B cell receptor-associated signaling in naïve and memory human B cells revealed by phosphoflow, a powerful technique to study signaling at the single cell level
Abstract
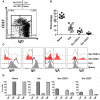
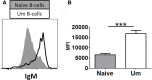
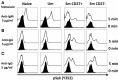
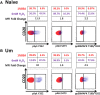
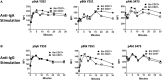
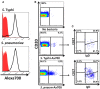
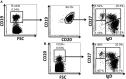
Following interaction with cognate antigens, B cells undergo cell activation, proliferation, and differentiation. Ligation of the B cell receptor (BCR) leads to the phosphorylation of BCR-associated signaling proteins within minutes of antigen binding, a process with profound consequences for the fate of the cells and development of effector immunity. Phosphoflow allows a rapid evaluation of various signaling pathways in complex heterogenous cell subsets. This novel technique was used in combination with multi-chromatic flow cytometry (FC) and fluorescent-cell barcoding (FCB) to study phosphorylation of BCR-associated signaling pathways in naïve and memory human B cell subsets. Proteins of the initiation (Syk), propagation (Btk, Akt), and integration (p38MAPK and Erk1/2) signaling units were studied. Switched memory (Sm) CD27+ and Sm CD27- phosphorylation patterns were similar when stimulated with anti-IgA or -IgG. In contrast, naïve and unswitched memory (Um) cells showed significant differences following IgM stimulation. Enhanced phosphorylation of Syk was observed in Um cells, suggesting a lower activation threshold. This is likely the result of higher amounts of IgM on the cell surface, higher pan-Syk levels, and enhanced susceptibility to phosphatase inhibition. All other signaling proteins evaluated also showed some degree of enhanced phosphorylation in Um cells. Furthermore, both the phospholipase C-γ2 (PLC-γ2) and phosphatidylinositol 3-kinase (PI3K) pathways were activated in Um cells, while only the PI3K pathway was activated on naïve cells. Um cells were the only ones that activated signaling pathways when stimulated with fluorescently labeled S. Typhi and S. pneumoniae. Finally, simultaneous evaluation of signaling proteins at the single cell level (multiphosphorylated cells) revealed that interaction with gram positive and negative bacteria resulted in complex and diverse signaling patterns. Phosphoflow holds great potential to accelerate vaccine development by identifying signaling profiles in good/poor responders.
Keywords: IgM memory B cells; Salmonella Typhi; Streptococcus pneumoniae; cell signaling; fluorescent-cell barcoding; naïve B cells; phosphoflow; vaccines.
Figures






Similar articles
-
Phosphoflow Protocol for Signaling Studies in Human and Murine B Cell Subpopulations.J Immunol. 2020 May 15;204(10):2852-2863. doi: 10.4049/jimmunol.1901117. Epub 2020 Apr 6. J Immunol. 2020. PMID: 32253241
-
Aberrant B cell receptor signaling in circulating naïve and IgA+ memory B cells from newly-diagnosed autoantibody-positive rheumatoid arthritis patients.J Autoimmun. 2024 Feb;143:103168. doi: 10.1016/j.jaut.2024.103168. Epub 2024 Feb 13. J Autoimmun. 2024. PMID: 38350168
-
Oral Challenge with Wild-Type Salmonella Typhi Induces Distinct Changes in B Cell Subsets in Individuals Who Develop Typhoid Disease.PLoS Negl Trop Dis. 2016 Jun 14;10(6):e0004766. doi: 10.1371/journal.pntd.0004766. eCollection 2016 Jun. PLoS Negl Trop Dis. 2016. PMID: 27300136 Free PMC article.
-
Regulation of the phospholipase C-gamma2 pathway in B cells.Immunol Rev. 2000 Aug;176:19-29. doi: 10.1034/j.1600-065x.2000.00605.x. Immunol Rev. 2000. PMID: 11043765 Review.
-
Regulation of B-cell activation and differentiation by the phosphatidylinositol 3-kinase and phospholipase Cgamma pathway.Immunol Rev. 2000 Aug;176:30-46. doi: 10.1034/j.1600-065x.2000.00611.x. Immunol Rev. 2000. PMID: 11043766 Review.
Cited by
-
Immunogenicity and Efficacy of Live-Attenuated Salmonella Typhimurium Vaccine Candidate CVD 1926 in a Rhesus Macaque Model of Gastroenteritis.Infect Immun. 2021 Sep 16;89(10):e0008721. doi: 10.1128/IAI.00087-21. Epub 2021 Jul 26. Infect Immun. 2021. PMID: 34310885 Free PMC article.
-
Naïve and memory B cells exhibit distinct biochemical responses following BCR engagement.Immunol Cell Biol. 2016 Sep;94(8):774-86. doi: 10.1038/icb.2016.41. Epub 2016 Apr 22. Immunol Cell Biol. 2016. PMID: 27101923
-
Precursor B-ALL Cell Lines Differentially Respond to SYK Inhibition by Entospletinib.Int J Mol Sci. 2021 Jan 8;22(2):592. doi: 10.3390/ijms22020592. Int J Mol Sci. 2021. PMID: 33435587 Free PMC article.
-
Changes in monocyte subsets in volunteers who received an oral wild-type Salmonella Typhi challenge and reached typhoid diagnosis criteria.Front Immunol. 2024 Aug 27;15:1454857. doi: 10.3389/fimmu.2024.1454857. eCollection 2024. Front Immunol. 2024. PMID: 39263222 Free PMC article.
-
Association of blood IgG with tumor necrosis factor-alpha and clinical course of chronic lymphocytic leukemia.EBioMedicine. 2018 Sep;35:222-232. doi: 10.1016/j.ebiom.2018.08.045. Epub 2018 Aug 30. EBioMedicine. 2018. PMID: 30174282 Free PMC article.
References
-
- Blair P. A., Noreña L. Y., Flores-Borja F., Rawlings D. J., Isenberg D. A., Ehrenstein M. R., et al. (2010). CD19+CD24hiCD38hi B Cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients. Immunity 32, 129–140 10.1016/j.immuni.2009.11.009 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

