NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia
- PMID: 23088210
- PMCID: PMC3613176
- DOI: 10.1089/ars.2012.4666
NADPH oxidase-mediated triggering of inflammasome activation in mouse podocytes and glomeruli during hyperhomocysteinemia
Abstract
Aim: Our previous studies have shown that NOD-like receptor protein (NALP3) inflammasome activation is importantly involved in podocyte dysfunction and glomerular sclerosis induced by hyperhomocysteinemia (hHcys). The present study was designed to test whether nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-mediated redox signaling contributes to homocysteine (Hcys)-induced activation of NALP3 inflammasomes, an intracellular inflammatory machinery in podocytes in vitro and in vivo.
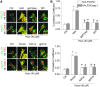
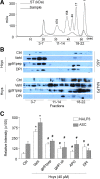
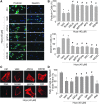
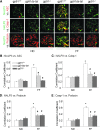
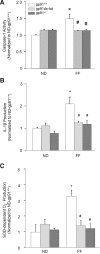
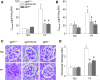
Results: In vitro confocal microscopy and size-exclusion chromatography revealed that upon NADPH oxidase inhibition by gp91(phox) siRNA, gp91ds-tat peptide, diphenyleneiodonium, or apocynin, aggregation of inflammasome proteins NALP3, apoptosis-associated speck-like protein (ASC), and caspase-1 was significantly attenuated in mouse podocytes. This NADPH oxidase inhibition also resulted in diminished Hcys-induced inflammasome activation, evidenced by reduced caspase-1 activity and interleukin-1β production. Similar findings were observed in vivo where gp91(phox-/-) mice and mice receiving a gp91ds-tat treatment exhibited markedly reduced inflammasome formation and activation. Further, in vivo NADPH oxidase inhibition protected the glomeruli and podocytes from hHcys-induced injury as shown by attenuated proteinuria, albuminuria, and glomerular sclerotic changes. This might be attributed to the fact that gp91(phox-/-) and gp91ds-tat-treated mice had abolished infiltration of macrophages and T-cells into the glomeruli during hHcys.
Innovation: Our study for the first time links NADPH oxidase to the formation and activation of NALP3 inflammasomes in podocytes.
Conclusion: Hcys-induced NADPH oxidase activation is importantly involved in the switching on of NALP3 inflammasomes within podocytes, which leads to the downstream recruitment of immune cells, ultimately resulting in glomerular injury and sclerosis.
Figures


References
-
- Cavalca V. Cighetti G. Bamonti F. Loaldi A. Bortone L. Novembrino C. De Franceschi M. Belardinelli R. Guazzi MD. Oxidative stress and homocysteine in coronary artery disease. Clin Chem. 2001;47:887–892. - PubMed
-
- Dai J. Wang X. Immunoregulatory effects of homocysteine on cardiovascular diseases. Sheng Li Xue Bao. 2007;59:585–592. - PubMed
-
- Edirimanne VE. Woo CW. Siow YL. Pierce GN. Xie JY. O K. Homocysteine stimulates NADPH oxidase-mediated superoxide production leading to endothelial dysfunction in rats. Can J Physiol Pharmacol. 2007;85:1236–1247. - PubMed
-
- Ernest S. Hosack A. O'Brien WE. Rosenblatt DS. Nadeau JH. Homocysteine levels in A/J and C57BL/6J mice: genetic, diet, gender, and parental effects. Physiol Genomics. 2005;21:404–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

