Bacillus coagulans GBI-30, 6086 limits the recurrence of Clostridium difficile-Induced colitis following vancomycin withdrawal in mice
- PMID: 23088680
- PMCID: PMC3484022
- DOI: 10.1186/1757-4749-4-13
Bacillus coagulans GBI-30, 6086 limits the recurrence of Clostridium difficile-Induced colitis following vancomycin withdrawal in mice
Abstract
Background: Recently, we found that the probiotic strain Bacillus coagulans GBI-30, 6086 (GanedenBC30) improved indices of Clostridium difficile (C. difficile)-induced colitis in mice (Fitzpatrick et al., Gut Pathogens, 2011). Our goal was to determine if BC30 could also prevent the recurrence of C. difficile-induced colitis in mice, following initial treatment with vancomycin. During study days 0 through 5, mice were treated with antibiotics. On day 6, the C. difficile strain VPI 10463 was given by oro-gastric gavage at ≈ 5x104 CFU to induce colitis. Mice were treated on study days 6 to 10 with vancomycin (50 mg/kg) (vanco) or vehicle (saline) by gavage. On days 10 to16, mice were dosed by gavage with saline vehicle or BC30 (2 x 109 CFU per day). Mice were monitored for mortality, weight loss and diarrhea. On study days 14, 16 and 17, stools and colons were collected for analyzing other parameters of colitis.
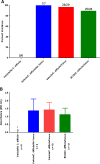
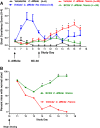
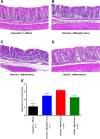
Results: The mean stool consistency score in Vehicle/C.difficile/Vanco mice increased from 0.4 (day 10) to a range of 1.1 to 1.4 (days 14 to 17), indicating the recurrence of colitis. On days 13 through 17, the stool consistency scores for the vancomycin/BC30 mice were significantly lower (p< 0.05) than for the vancomycin/vehicle cohort of animals. On day 17, 88.9% of mice treated with BC30 had normal stools, while this value was 0% with vehicle treatment (p value = 0.0004). Colonic myeloperoxidase (Units/2 cm colon) was significantly (p < 0.05) reduced from 4.3 ± 0.7 (Vehicle/C.difficile/Vanco) to 2.6 ± 0.2 (BC30/C. Difficle/Vanco). The colonic histology score and Keratinocyte derived-chemokine level in the colon were also lower in BC30 treated mice.
Summary: In BC30-treated mice, there was evidence of better stool consistency, as well as improved biochemical and histological indices of colitis, following initial treatment of animals with vancomycin.
Conclusion: BC30 limited the recurrence of CD-induced colitis following vancomycin withdrawal in mice.
Figures



References
-
- Pothoulakis C, Lamont JT. Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins. Am J Gastrointest Liver Physiol. 2001;28:G178–G183. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

