Continuous controllable balloon dilation: a novel approach for cervix dilation
- PMID: 23088906
- PMCID: PMC3543240
- DOI: 10.1186/1745-6215-13-196
Continuous controllable balloon dilation: a novel approach for cervix dilation
Abstract
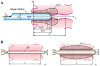
Background: Cervical dilation using mechanical dilators is associated with various complications, such as uterine perforation, cervical laceration, infections and intraperitoneal hemorrhage. To achieve safe and painless cervical dilation, we constructed a new medical device to achieve confident mechanical cervical dilation: a continuous controllable balloon dilator (CCBD).
Methods: Controlled pumping of incompressible fluid into the CCBD increases the pressure and outer diameter of the CCBD, continuously dilating the cervical canal. The reliability of the CCBD was confirmed in vitro (testing for consistency and endurance, with no detected risk for breakage) and in vivo. A multi-center clinical study was conducted,with 120 pregnant women randomly assigned to one of three groups: Group I,control group, no dilation;Group II,mechanical dilation, Hegar dilator (HeD); and Group III,CCBD. The tissue material for histological evaluation was obtained from the endocervical mucosa before and after dilation using the HeD or CCBD.
Results: The CCBD dilations were successful and had no complications in all 40 patients of Group III. The cervical tissue was markedly less damaged after CCBD dilation compared with HeD dilation (epithelium damage: 95% (HeD) vs. 45% (CCBD), P <0.001; basal membrane damage: 82.5% (HeD) vs. 27.5% (CCBD), P <0.001; stromal damage: 62.5% (HeD) vs. 37.5% (CCBD), P <0.01). Cervical hemorrhagia was observed in 90% of the patients after HeD dilation versus in 32.5% of the patients after CCBD dilation.
Conclusions: The CCBD should be used as a replacement for mechanical dilators to prevent uterine and cervical injury during cervical dilation.
Trial registration: ISRCTN54007498.
Figures

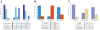
References
-
- Fox MC, Hayes JL. Cervical preparation for second-trimester surgical abortion prior to 20 weeks of gestation. Contraception. 2007;76:486–495. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

