Glycine treatment of the risk syndrome for psychosis: report of two pilot studies
- PMID: 23089076
- PMCID: PMC4028140
- DOI: 10.1016/j.euroneuro.2012.09.008
Glycine treatment of the risk syndrome for psychosis: report of two pilot studies
Abstract
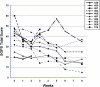
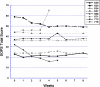
Patients meeting criteria for the risk syndrome for psychosis have treatment needs including positive and negative symptoms and cognitive impairment. These features could potentially respond to NMDA glycine-site agonists. The present objective was to determine which symptoms or domains of cognition promise to show the greatest response to glycine in risk syndrome patients. We conducted two short-term pilot studies of glycine used without adjunctive antipsychotic medication. In the first trial, 10 risk syndrome subjects received open-label glycine at doses titrated to 0.8 g/kg/d for 8 weeks, followed by discontinuation and 16 weeks of evaluation for durability of effects. In the second, 8 subjects were randomized to double-blind glycine vs. placebo for 12 weeks, followed by open-label glycine for another 12 weeks. Patients were evaluated every 1-2 weeks with the Scale Of Psychosis-risk Symptoms (SOPS) and before and after treatment with a neurocognitive battery. Within-group and between-group effect sizes were calculated. Effect sizes were large for positive (open-label within-group -1.10, double-blind between-group -1.11) and total (-1.39 and -1.15) symptoms and medium-to-large (-0.74 and -0.79) for negative symptoms. Medium or large effect sizes were also observed for several neurocognitive measures in the open-label study, although data were sparse. No safety concerns were identified. We conclude that glycine was associated with reduced symptoms with promising effect sizes in two pilot studies and a possibility of improvement in cognitive function. Further studies of agents that facilitate NMDA receptor function in risk syndrome patients are supported by these preliminary findings.
Trial registration: ClinicalTrials.gov NCT00268749 NCT00291226.
Keywords: Glycine; NMDA receptor; Prodrome; Psychosis; Risk syndrome; Schizophrenia.
Copyright © 2012 Elsevier B.V. and ECNP. All rights reserved.
Figures
Similar articles
-
The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments.Am J Psychiatry. 2007 Oct;164(10):1593-602. doi: 10.1176/appi.ajp.2007.06081358. Am J Psychiatry. 2007. PMID: 17898352 Clinical Trial.
-
Sarcosine or D-serine add-on treatment for acute exacerbation of schizophrenia: a randomized, double-blind, placebo-controlled study.Arch Gen Psychiatry. 2005 Nov;62(11):1196-204. doi: 10.1001/archpsyc.62.11.1196. Arch Gen Psychiatry. 2005. PMID: 16275807 Clinical Trial.
-
The effects of glycine on auditory mismatch negativity in schizophrenia.Schizophr Res. 2018 Jan;191:61-69. doi: 10.1016/j.schres.2017.05.031. Epub 2017 Jun 9. Schizophr Res. 2018. PMID: 28602646 Clinical Trial.
-
Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site.Eur Arch Psychiatry Clin Neurosci. 2008 Feb;258(1):16-27. doi: 10.1007/s00406-007-0757-8. Epub 2007 Sep 27. Eur Arch Psychiatry Clin Neurosci. 2008. PMID: 17901997 Review.
-
Early psychotic experiences: Interventions, problems and perspectives.Psychiatriki. 2015 Jan-Mar;26(1):45-54. Psychiatriki. 2015. PMID: 25880383 Review.
Cited by
-
Managing Negative Symptoms of Schizophrenia: How Far Have We Come?CNS Drugs. 2017 May;31(5):373-388. doi: 10.1007/s40263-017-0428-x. CNS Drugs. 2017. PMID: 28397113 Review.
-
Comparative Pro-cognitive and Neurochemical Profiles of Glycine Modulatory Site Agonists and Glycine Reuptake Inhibitors in the Rat: Potential Relevance to Cognitive Dysfunction and Its Management.Mol Neurobiol. 2020 May;57(5):2144-2166. doi: 10.1007/s12035-020-01875-9. Epub 2020 Jan 20. Mol Neurobiol. 2020. PMID: 31960362 Free PMC article.
-
Interventions for prodromal stage of psychosis.Cochrane Database Syst Rev. 2019 Nov 1;2019(11):CD012236. doi: 10.1002/14651858.CD012236.pub2. Cochrane Database Syst Rev. 2019. PMID: 31689359 Free PMC article.
-
Altering the course of schizophrenia: progress and perspectives.Nat Rev Drug Discov. 2016 Jul;15(7):485-515. doi: 10.1038/nrd.2016.28. Epub 2016 Mar 4. Nat Rev Drug Discov. 2016. PMID: 26939910 Review.
-
Modulating NMDA Receptor Function with D-Amino Acid Oxidase Inhibitors: Understanding Functional Activity in PCP-Treated Mouse Model.Neurochem Res. 2016 Feb;41(1-2):398-408. doi: 10.1007/s11064-016-1838-8. Epub 2016 Feb 8. Neurochem Res. 2016. PMID: 26857796 Free PMC article.
References
-
- Amminger GP, Schafer MR, Papageorgiou K, Klier CM, Cotton SM, Harrigan SM, Mackinnon A, McGorry PD, Berger GE. Long-chain omega-3 fatty acids for indicated prevention of psychotic disorders: a randomized, placebo-controlled trial. Arch. Gen. Psychiatry. 2010;67:146–154. - PubMed
-
- Buchanan RW, Javitt DC, Marder SR, Schooler NR, Gold JM, McMahon RP, Heresco-Levy U, Carpenter WT. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): the efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am. J. Psychiatry. 2007;164:1593–1602. - PubMed
-
- Carlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia—therarpeutic implications. Biol. Psychiatry. 1999;46:1388–1395. - PubMed
-
- Carpenter WT, van Os J. Should attenuated psychosis syndrome be a DSM-5 diagnosis? Am. J. Psychiatry. 2011;168:460–463. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

