Human type 2 myeloid dendritic cells produce interferon-λ and amplify interferon-α in response to hepatitis C virus infection
- PMID: 23089201
- PMCID: PMC3568254
- DOI: 10.1053/j.gastro.2012.10.034
Human type 2 myeloid dendritic cells produce interferon-λ and amplify interferon-α in response to hepatitis C virus infection
Abstract
Background & aims: The type III interferons (IFN-λs: interleukin [IL]-28a, IL-28b, and IL-29) have important roles in hepatitis C virus (HCV) infection, but little is understood about what cells produce these cytokines or how production is activated. We investigated whether human immune cells recognize HCV-infected cells and respond by producing IFN-λ.
Methods: We cultured healthy human peripheral blood mononuclear cells (PBMCs) with different populations of immune cells and Japanese fulminant hepatitis-1 (JFH-1) HCV-infected Huh7.5 (cell culture-derived HCV particles [HCVcc]/Huh7.5) cells.
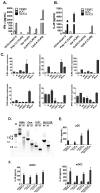
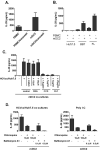
Results: Human PBMCs recognized HCVcc/Huh7.5 cells and responded by producing IFN-α, IFN-γ, and IFN-λ. A rare subset of myeloid dendritic cells (mDCs), which are blood DC antigen (BDCA)+ (also called mDC2 cells), were the major source of IL-28 and IL-29 production in response to HCVcc/Huh7.5 cells. Plasmacytoid DCs produced IFN-α, whereas natural killer and natural killer T cells were the main source of IFN-γ production in co-culture experiments. Of the endosomal Toll-like receptors (TLRs)3, 7, 8, and 9, only TLR3 or double-stranded HCV RNA induced production of IL-28 and IL-29 by mDC2s; endosomal maturation was required. Production of IFN-α and IFN-λ were linked-IFN-λ increased production of IFN-α by plasmacytoid DCs and IFN-α significantly increased production of IFN-λ.
Conclusions: mDC2s are a major source of IFN-λ production by PBMCs in response to HCVcc/Huh7.5 cells. mDC2s are activated through the TLR3 pathway, indicating that human DCs efficiently can initiate an immune response against HCV infection. IFN-λ therefore has an important role in HCV infection.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

